Researchers say they have used Artificial Intelligence and citizen-submitted photos to identify what they believe was the first Anopheles stephensi detected in Madagascar, amid a rising threat from the malaria-transmitting mosquito…

Researchers say they have used Artificial Intelligence and citizen-submitted photos to identify what they believe was the first Anopheles stephensi detected in Madagascar, amid a rising threat from the malaria-transmitting mosquito…
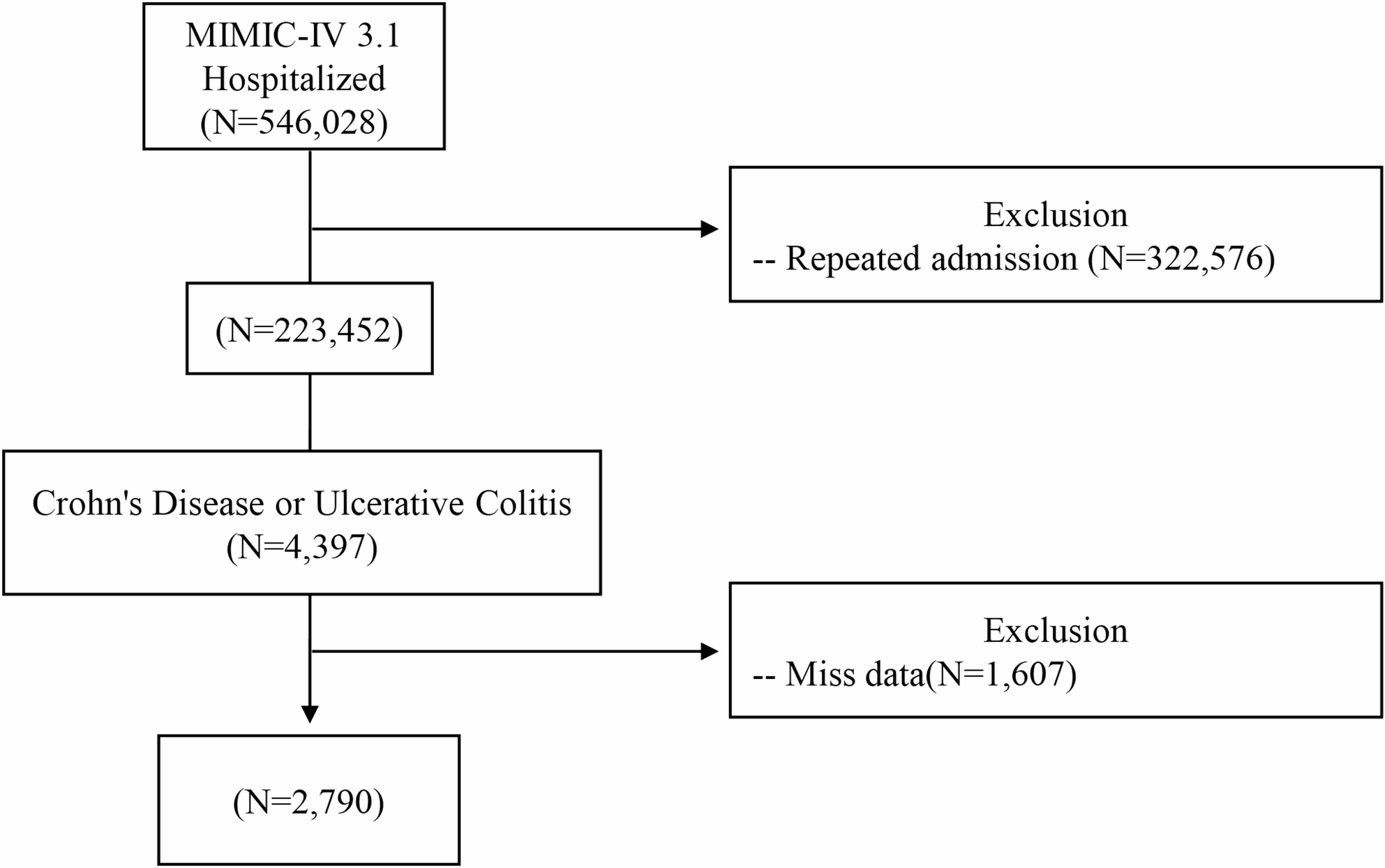
This retrospective cohort study was conducted using data from the Medical Information Mart for Intensive Care IV (MIMIC-IV) V.3.1 Database, 2008–2022 which is a comprehensive, publicly available critical care dataset…
The details are fuzzy, but I think my husband and I downloaded the YouTube Kids app to our TV sometime in 2022, when between one and three members of the household came down with the flu at the same time. Like countless parents of toddlers before…

Dutch government’s decision to relinquish control of chipmaker comes after major disruption to automotive supply chains.
Published On 20 Nov 2025
The Netherlands has announced that it will return control of chipmaker Nexperia to its Chinese parent company, a step towards resolving a standoff between The Hague and Beijing that upended automotive supply chains.
Dutch Economic Affairs Minister Vincent Karremans said on Wednesday that he had suspended an order to effectively seize control of the chipmaker following “constructive” talks with Chinese officials and consultations with European and international partners.
list of 4 itemsend of list
“We are positive about the measures already taken by the Chinese authorities to ensure the supply of chips to Europe and the rest of the world,” Karremans said in a statement.
“We see this as a show of goodwill. We will continue to engage in constructive dialogue with the Chinese authorities in the period ahead.”
China’s Ministry of Commerce welcomed the announcement as a “first step”, but called for the full revocation of the order, describing it as the “root cause” of the supply chain disruptions.
It also criticised a Dutch court’s “erroneous ruling” last month that forced out Nexperia’s Chinese CEO, Zhang Xuezheng, over alleged mismanagement.
Jo Van Biesebroeck, an economics professor at KU Leuven, said Europe’s efforts to craft a strategy for managing China’s involvement in critical supply chains were a “work in progress”.
“The Nexperia action was triggered by specific actions, and the main worry now seems to be diminished with the personnel change at Nexperia,” Biesebroeck told Al Jazeera.
“The Dutch government made clear how far it is willing to go, and it seems like China has met them halfway.”
The Dutch government took effective control of Nexperia, owned by Jiaxing-based Wingtech, in late September, citing the need to ensure chip supplies amid concerns Zhang could move manufacturing operations and intellectual property to China.
The move came after the United States had warned the Netherlands that the company would likely be placed on its list of sanctioned firms unless it replaced Zhang, though Dutch officials have denied acting due to pressure from Washington.
Beijing condemned the Dutch government’s intervention, invoked under the Cold War-era Goods Availability Act, as an act of “improper interference” in a company’s affairs and blocked exports of some Nexperia products manufactured in China in response.
Japanese carmakers Honda and Nissan were forced to cut back production amid the resulting disruption to supply chains, while Germany’s Mercedes-Benz announced that it had taken steps to secure chip supplies in the short term.
Chinese authorities lifted the ban on Nexperia exports earlier this month as part of measures agreed to under the trade truce announced by US President Donald Trump and Chinese leader Xi Jinping last month in South Korea.
Gonorrhea is becoming increasingly resistant to antibiotics, according to

A tech company has ambitious goals and tells its employees they should be ready for some hardcore work.
No, I’m not talking about Amazon. Or Nvidia. Or Tesla.
This time around, it’s Tools for Humanity, Sam Altman’s…

Family caregiving is having a moment in the spotlight.
Just in time for National Family Caregivers Month, Marvel star Chris Hemsworth is releasing a new documentary, A Road Trip to Remember,…

The proven expertise of the Rolls-Royce LibertyWorks team – in areas like subsonic, supersonic, and hypersonic propulsion; electrical power; thermal management; and mobile nuclear power – has shaped technology solutions applicable to a wide range of missions and customers.
LibertyWorks has worked with technology organizations of the U.S. Air Force, U.S. Army, U.S. Navy, as well as the Defense Advanced Research Projects Agency, NASA, and others to transform concepts to realities for three decades.
The LibertyWorks team developed advanced technologies contributing to the Short Take-Off and Vertical Landing (STOVL) capabilities of the LiftSystem used in the Technology Demonstrator – forerunner to the revolutionary U.S. Marine Corps F-35B.
The team also developed technologies supporting platforms like the subsonic U.S. Navy MQ-25 Stingray autonomous refueling platform and the new U.S. Army MV-75 Future Long Range Assault Aircraft, and continues work in areas like air breathing hypersonic propulsion to support future needs. LibertyWorks is also proud to be part of the U.S. Department of War’s Project Pele advanced nuclear microreactor.
To support LibertyWorks and its other U.S. defense operations, Rolls-Royce North America has invested more than $1 billion in technology enhancements, facility upgrades and test capabilities in Indianapolis over the past decade.
In the United States, Rolls-Royce employs more than 5,000 people and supports hundreds of American suppliers in 34 locations across 26 states. Rolls-Royce operations contributed $6.2 billion to the U.S. economy in 2024.
Breaking News
|
GLOBAL-SWEDEN
|