ATLANTA — The music artist and entertainment executive Usher is suing a group of investors who have been trying to open a new restaurant and lounge in Atlanta.
Usher Raymond IV lent $1.7 million to the investor group toward the purchase of…

ATLANTA — The music artist and entertainment executive Usher is suing a group of investors who have been trying to open a new restaurant and lounge in Atlanta.
Usher Raymond IV lent $1.7 million to the investor group toward the purchase of…
JUANA SUMMERS, HOST:
Weight loss drugs like Wegovy and Zepbound are helping millions of Americans shed pounds, but these drugs affect the brain in ways that can cause nausea and other side effects. NPR’s…
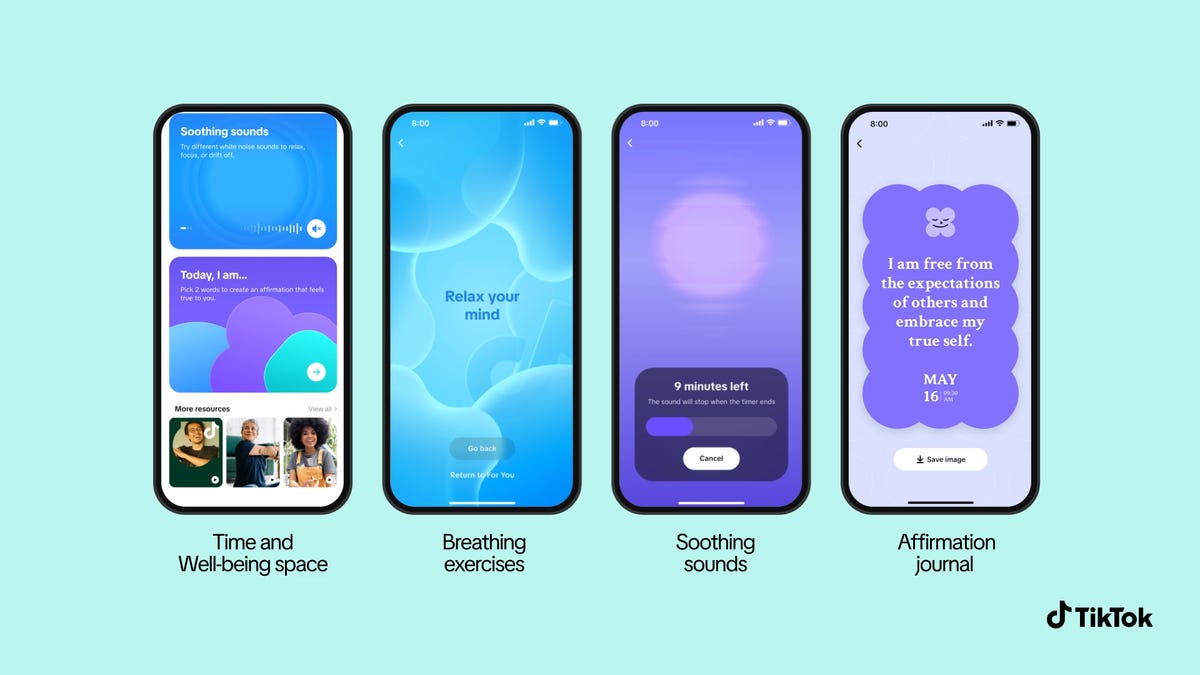
Doomscrolling, the nickname given to constantly reading (usually stressful) content on our always-available phones, can ruin our sleep and apparently cause bathroom-related issues. But it can be tough to put those phones down.TikTok announced on…
WASHINGTON — Even Australia’s most protected marine habitats are likely to suffer extreme impacts from climate change by 2040. According to a new study, even under today’s optimistic climate…

More motor sport history was made when Macau staged the inaugural FIA F4 World Cup last weekend. This is what the winner, 19-year-old Jules Roussel from France, had to say.
For a young driver, how…

The upper helps with that disappearing act, too. It’s the type of upper I wish every daily trainer had; padded where it needs to be (around the ankle) but thin and breathable over the midfoot and forefoot. It feels soft and secure without…

The Oscars are making room for one more award on their live broadcast in 2026. The new prize for achievement in casting will be part of the 98th Academy Awards in March, the academy told The Associated Press on Wednesday.
That brings the total…

United States President Donald Trump has pledged to address the conflict in Sudan, in response to an apparent request from Saudi Crown Prince Mohammed bin Salman.
On Wednesday, Trump announced his intention to intervene twice, once at the Saudi…

Nematologists at the University of California, Davis, including Valerie Williamson , professor emerita in the Department of Plant Pathology and associate professor Shahid Siddique , Department of Entomology and Nematology, have long…