Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
The Nintendo Switch 2 will appear on many a festive-season wish list this year. Already, strong sales of the gaming…

Unlock the Editor’s Digest for free
Roula Khalaf, Editor of the FT, selects her favourite stories in this weekly newsletter.
The Nintendo Switch 2 will appear on many a festive-season wish list this year. Already, strong sales of the gaming…
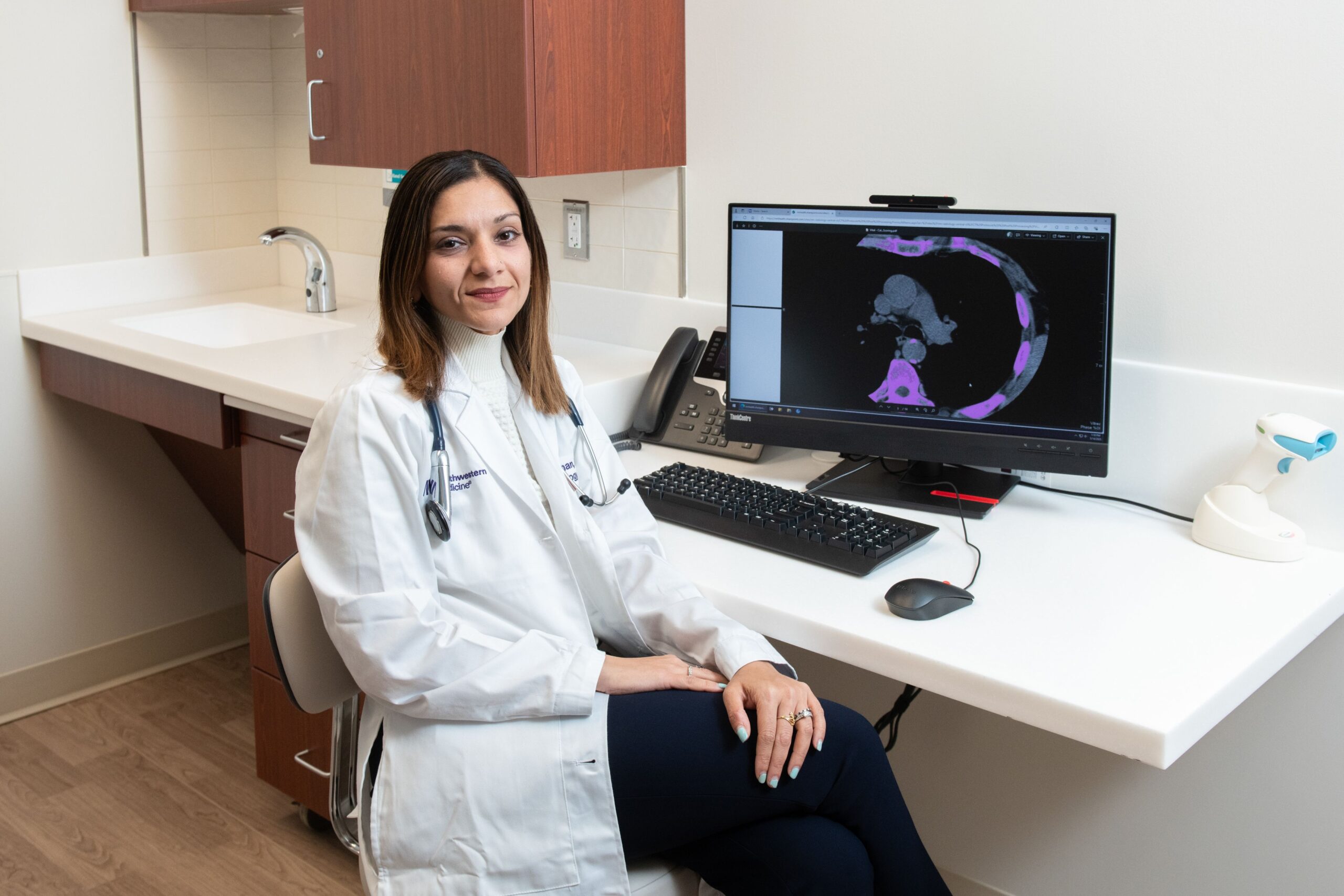
Northwestern University researchers have developed the first percentile-based tool for communicating 30-year
The…

Diane Ladd’s cause of death has come to light, weeks after the three-time Oscar-nominated “Rambling Rose” and “Wild at Heart” star died at age 89.
The actor died of “acute on chronic hypoxic respiratory failure,” according to her…


A pair of stunning extra-time goals from Kieran Tierney and Kenny McLean gave Scotland a 4-2 win over Denmark on the last day of UEFA qualifying and a spot in the World Cup for the first time since 1998.
Scott McTominay opened proceedings in the…

SAVE $34.01: A 4-pack of Apple AirTags is on sale at Amazon for $64.99, down from the normal price of $99. That’s a 34% discount and just 50 cents more than the lowest we’ve ever…

We are not advocates of executing people in cruel and unusual ways here on Futurism, but we have to admit we are intrigued by this…