Archaeologists working in Crimea have recovered ancient DNA from a tiny bone and matched it to Neanderthals from Siberia. The genetic link spans about 1,900 miles across Eurasia and dates to roughly 45,000 years ago.
The result shows that…
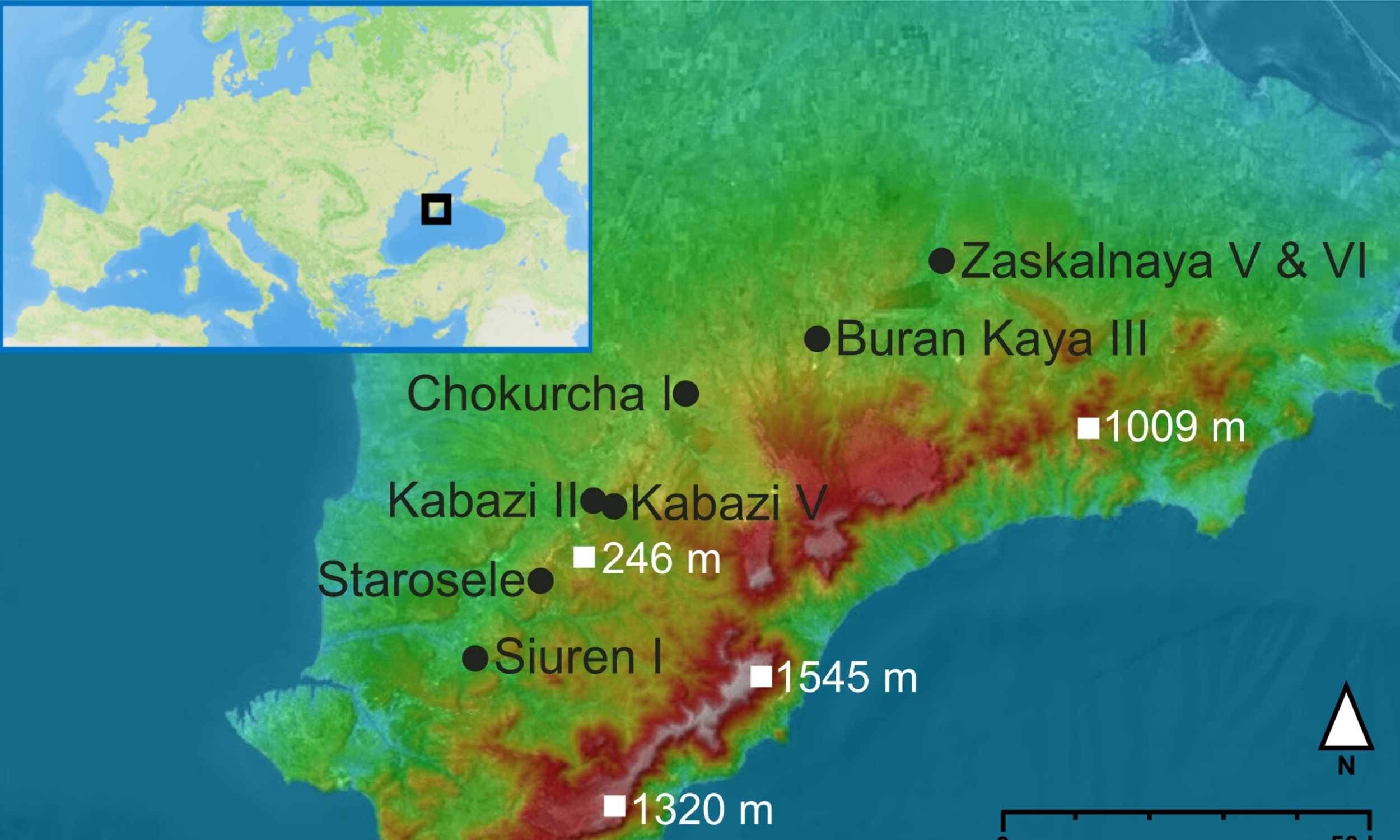
Archaeologists working in Crimea have recovered ancient DNA from a tiny bone and matched it to Neanderthals from Siberia. The genetic link spans about 1,900 miles across Eurasia and dates to roughly 45,000 years ago.
The result shows that…

Troy Parrott’s brilliant hat-trick, his third coming in the depths of injury time, delivered the Republic of Ireland into the World Cup playoffs, denying a sickened Hungary at the death. To cap a remarkable week in Irish football history,…

SYDNEY, Nov. 17, 2025 (GLOBE NEWSWIRE) — Halfway through its end-of-year Tick It Off Fest, SHEIN is proving that resolutions aren’t just for January. Across TikTok and Instagram, Australians are sharing their biggest wins of the year -…

Tehran is willing to restart nuclear talks with Washington as long as it is treated with “dignity and respect”, Iran’s foreign minister has told the Guardian.
Abbas Araghchi said only diplomacy worked, and disclosed fresh requests had come…
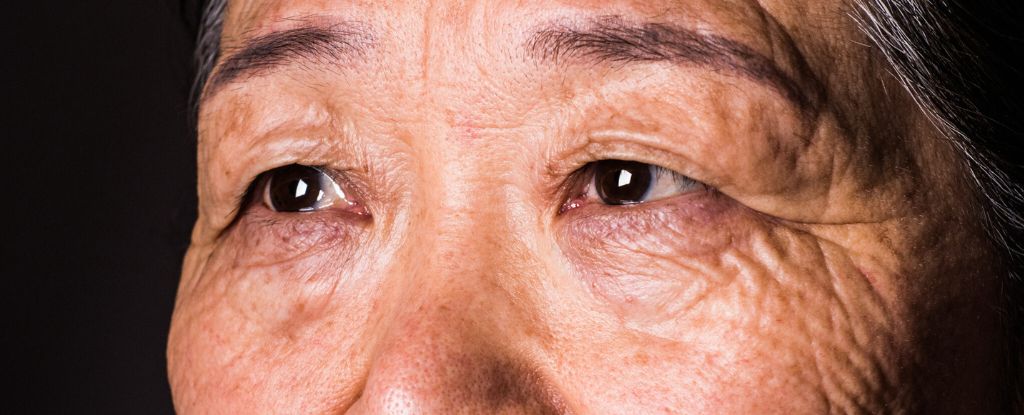
The eyes are a window to the brain – and this outward extension of the central nervous system may reveal early signs of cognitive decline.
Two recent and large population studies, one in the United Kingdom and another in Australia, suggest…

AO LIVE Opening Week features:
Hot Chip [LIVE] – Tuesday 13 January 2026 – 5:30pm
+ Milo Eastwood and O Honey
The much loved British synth-pop outfit returns to celebrate their new Best Of album, Joy in Repetition —…

BROOKINGS, S.D. – North Dakota State wrestling’s Devin Wasley and Ezekiel Witt led the Bison at the…

Tetra Tech, Inc. recently announced record financial results for the fourth quarter of fiscal 2025, reporting quarterly sales of US$1,330.1 million and net income of US$127.75 million, along with the declaration of a quarterly dividend of US$0.065 per share payable on December 12, 2025.
Management highlighted a robust backlog of US$4.14 billion and strong demand for water and environmental services, underpinned by large new contract wins and guidance anticipating further earnings growth in 2026 despite sector funding shifts.
We’ll explore how Tetra Tech’s record operating margins and backlog expansion could reshape the company’s investment narrative for long-term investors.
The latest GPUs need a type of rare earth metal called Neodymium and there are only 37 companies in the world exploring or producing it. Find the list for free.
For investors considering Tetra Tech, the core thesis centers on the company’s ability to maintain strong earnings growth and margin expansion as it shifts focus to high-value water and environmental services contracts. The most recent record quarterly results and robust backlog reinforce the short-term catalyst of high-margin government service wins, but do little to address the growing risk around revenue concentration and the uncertainties created by lapsed USAID and Department of State contracts.
Among the latest announcements, the $249 million contract award from the U.S. Army Corps of Engineers stands out as directly supporting future backlog and near-term revenue, tying in closely to the key catalyst of government infrastructure spending highlighted in company guidance. This new pipeline helps offset concerns about lower contributions from previous episodic disaster response work.
However, investors should not overlook that, while government contract wins support revenue visibility, the company still faces the risk that shifts in federal funding priorities could result in…
Read the full narrative on Tetra Tech (it’s free!)
Tetra Tech’s narrative projects $4.7 billion in revenue and $559.6 million in earnings by 2028. This requires a 0.8% annual revenue decline and a $343.5 million earnings increase from current earnings of $216.1 million.
Uncover how Tetra Tech’s forecasts yield a $42.60 fair value, a 18% upside to its current price.
Four individual fair value estimates from the Simply Wall St Community range from US$23.16 to US$42.60 per share. Despite renewed optimism from recent federal contract wins, some see continued risk around margin volatility and revenue concentration that could shape long-term outlooks.
