The Security Council today extended the mandate of the United Nations Multidimensional Integrated Stabilization Mission in the Central African Republic (MINUSCA) until 15 November 2026.
By a recorded vote of 14 in favour and 0 against, with 1…
The Security Council today extended the mandate of the United Nations Multidimensional Integrated Stabilization Mission in the Central African Republic (MINUSCA) until 15 November 2026.
By a recorded vote of 14 in favour and 0 against, with 1…
The following is a near-verbatim transcript of today’s briefing by Stéphane Dujarric, Spokesman for the Secretary-General.
**Briefing
Good afternoon. Just a reminder, at 1:30 p.m., in this very room, you will be hearing from Philippe…

The world is producing more oil than it can use and by next year there could be a glut of 4m excess barrels a day entering the market, according to the global energy watchdog.
The International Energy Agency said the surplus in 2026 was likely to be larger than previously forecast, despite a decision from the biggest oil producers to pause their plan to increase crude exports.
The Paris-based agency, which was set up after the 1973 oil crisis to monitor global supplies, pointed to a slower-than-usual growth in the world’s oil demand to explain the growing glut.
“Global oil market balances are looking increasingly lopsided, as world oil supply is forging ahead while oil demand growth remains modest by historical standards,” the IEA said.
The warning of a looming oversupply has emerged in the same week that the agency published its energy outlook report, including a controversial scenario in which global oil demand would continue to grow until 2050.
The model, which takes a conservative view of global climate action, was dropped in 2020 after the IEA was repeatedly criticised for underestimating the growth of renewable energy in its annual report.
The IEA returned the scenario to its outlook this year, as leaders gathered in Belém, in Brazil, for the Cop30 climate talks, after calls from the White House to present a more optimistic view for the future of oil. The IEA has denied it reintroduced its new scenario in response to pressure from the US.
Critics of the forecast believe it underestimates the pace of electric vehicle take-up, particularly in developing countries in Asia, a trend that is already helping to reduce the world’s demand for oil.
The IEA’s report also included two main scenarios in which oil consumption reached a peak by 2030 because of the strong take-up onof electric vehicles and renewable energy.
In all scenarios, renewable energy is expected to at least double over the next five years. The outlook suggested the world was likely to build more renewable energy projects in the next five years than had been rolled out over the past 40.
The Global Wind Energy Council (GWEC) said there was now “irreversible momentum towards the age of electricity”, which in every IEA scenario showed that renewable energy was “growing faster than any other major energy source”.
A GWEC spokesperson said: “It is important an outdated political narrative does not distract from this data-driven reality.”
In its monthly report the IEA said it now expected global oil supply to grow by about 3.1m barrels per day (bpd) in 2025, and by 2.5m next year, each up by about 100,000 bpd on the month.
With supply outpacing demand, the IEA’s latest monthly oil report suggested that by 2026 total oil supply would be 4.09m bpd higher than total demand, up from an implied surplus of 3.97m bpd in last month’s report.

Primary Sjögren’s syndrome (pSS) is a chronic progressive autoimmune disease characterized by lymphocytic infiltration of the exocrine glands, commonly manifesting as dryness in the mouth and eyes.1 Because of long-term chronic…

Duncan Regional Hospital Becomes First in U.S. to Implement BD Alaris™ EMR Infusion Interoperability with MEDITECH
FRANKLIN LAKES, N.J., Nov. 13, 2025 /PRNewswire/ — BD (Becton, Dickinson and Company) (NYSE: BDX), a leading global medical technology company, today announced that Duncan Regional Hospital (“DRH Health”) has become the first hospital in the United States to implement BD Alaris™ EMR (Electronic Medical Record) Interoperability with the MEDITECH electronic health record (EHR), marking a significant advancement in patient safety, workflow efficiency and clinician support.
This integration brings together BD’s robust market-leading bi-directional infusion interoperability – now live at more than 960 sites in the United States. By expanding this capability to MEDITECH, BD now offers its Alaris™ EMR Interoperability solution to all three of the leading EHR systems in the U.S. market, providing advanced integration to an even broader range of healthcare organizations nationwide.
“Providing a safer environment for our patients is always our top priority at Duncan,” said Roger L. Neal, Vice President and Chief Operating Officer of DRH Health in Duncan, Okla. “With the current nursing shortage, this integration eliminates many small, repetitive tasks our nurses perform every day, allowing them to spend more time with patients and improving efficiency across our health system. We’re proud to lead the way as the first hospital in the nation to implement BD Alaris™ EMR Interoperability with MEDITECH. This integration brings important safety features to one of the most complex processes in healthcare, and we’re confident it will result in safer administration, better documentation, more consistent infusion workflows, and greater satisfaction for our nursing teams and most importantly our patients.”
Enhancing Patient Safety and Efficiency
BD Alaris™ EMR Interoperability enables seamless connection of infusion pumps into MEDITECH’s Patient Care and Patient Safety solution. Clinicians can now scan to verify patients, medications and smart pumps; send infusion orders directly from MEDITECH to BD Alaris™ pumps; and receive infusion statuses back into the EHR. By reducing manual programming and error-prone steps, this solution supports comprehensive, near real-time documentation, and helps ensure the right patient receives the right medication at the right dose while improving safety across the board.
“We are excited to support Duncan Regional Hospital as the first in the U.S. to go live with BD Alaris™ EMR Interoperability integrated with MEDITECH,” said Tiffany Murphy, U.S. General Manager and Vice President of Medication Management Solutions. “This expansion marks a pivotal milestone, enabling us to offer our market-leading interoperability to a broader segment of healthcare organizations. With close to 1,000 live sites and a proven record of activating 12 new sites each month, BD is committed to advancing infusion safety and operational efficiency for hospitals across all three of the nation’s top EMRs.”
“This collaboration demonstrates our shared commitment to advancing patient safety and improving operational efficiency for clinicians,” said Emily Pacheco-Valente, RN, Product Manager at MEDITECH. “By connecting BD Alaris™ with MEDITECH’s robust HER platform, we are delivering many additional safety features to one of the most complex processes in healthcare – features hospitals can’t afford to overlook. We look forward to seeing the positive impact for both caregivers and patients.”
About BD Alaris™ EMR Interoperability
BD Alaris™ EMR Interoperability enables healthcare organizations to standardize infusion workflows, reduce inefficiencies, and minimize error-prone manual steps. By seamlessly connecting infusion pumps to EMR systems, the solution helps decrease the risk of IV medication errors and extends safety to every infusion administered. Learn more about BD Alaris™ EMR Interoperability: https://www.bd.com/en-ca/products-and-solutions/solutions/interoperability.
About BD
BD is one of the largest global medical technology companies in the world and is advancing the world of health by improving medical discovery, diagnostics, and the delivery of care. The company supports the heroes on the frontlines of health care by developing innovative technology, services and solutions that help advance both clinical therapy for patients and clinical processes for health care providers. BD and its more than 70,000 employees have a passion and commitment to help enhance the safety and efficiency of clinicians’ care delivery process, enable laboratory scientists to accurately detect disease and advance researchers’ capabilities to develop the next generation of diagnostics and therapeutics. BD has a presence in virtually every country and partners with organizations around the world to address some of the most challenging global health issues. By working in close collaboration with customers, BD can help enhance outcomes, lower costs, increase efficiency, improve safety, and expand access to health care. For more information on BD, please visit bd.com or connect with us on LinkedIn at www.linkedin.com/company/bd1/, X (formerly Twitter) @BDandCo or Instagram @becton_dickinson.
About DRH Health
DRH Health is a progressive, not-for-profit community hospital located in Duncan, Oklahoma, serving patients across the region since 1977. With a team of more than [1,000] dedicated healthcare professionals, DRH Health is committed to delivering compassionate, personalized care and exceeding the expectations of those we serve. We offer a seamless, state-of-the-art health services environment designed to meet the evolving needs of our community and make every patient ideal encounter a reality. Through continuous innovation and an unwavering commitment to excellence, DRH Health strives to improve patient outcomes and deliver exceptional healthcare experiences for patients and their families. For more information, visit www.drhhealth.org.
About MEDITECH
MEDITECH empowers healthcare organizations to expand their vision of what’s possible with Expanse, the intelligent EHR platform you can trust. Expanse transforms care and ushers health systems of all sizes into the future with AI-infused solutions, personalized workflows, next-level interoperability, and predictive analytics — all working together to drive better outcomes. See why thousands of healthcare organizations in 29 countries and territories have chosen Expanse. Visit ehr.meditech.com and follow us on YouTube, LinkedIn, X/Twitter, Facebook, Instagram, and Threads.
|
Contacts: Media: |
|
|
For BD: |
|
|
Fallon McLoughlin |
Adam Reiffe |
|
Director, Public Relations |
VP, Investor Relations |
|
201.258.0361 |
201.847.6927 |
|
fallon.mcloughlin@bd.com |
adam.reiffe@bd.com |
|
For DRH Health: |
|
|
Cyndi Crook |
|
|
Executive Director, DRH Foundation/Community Relations |
|
|
580-251-8844 |
|
|
cyndi.crook@drhhealth.org |
|
|
For MEDITECH: |
|
|
Jason Patnode |
|
|
Manager, Content, Branding, and Digital Marketing |
|
|
781-774-5746 |
|
|
jpatnode@meditech.com |

SOURCE BD (Becton, Dickinson and Company)
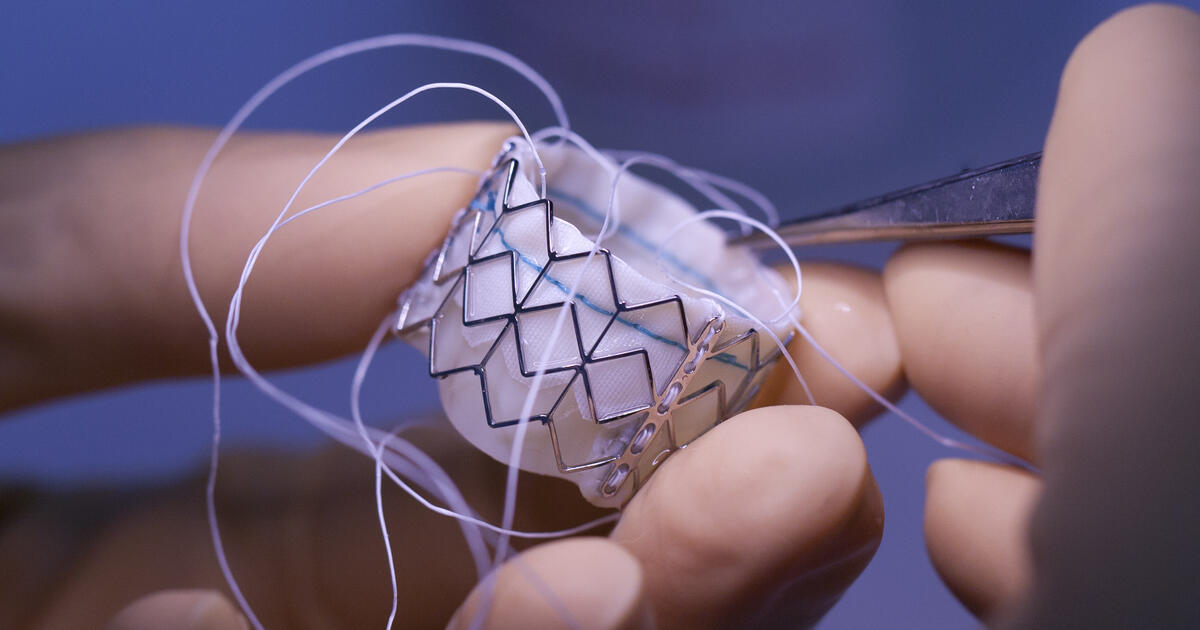
The Paris Central Division of the Unified Patent Court (UPC) ruled (40-page / 1.68MB PDF) that companies in the Meril Life Sciences group (Meril) infringed a European patent relating to a prosthetic heart valve and a delivery assembly. The patent, a third-generation divisional patent, belongs to US-based Edwards Lifesciences Corporation (Edwards). The patent was found to be valid as amended by an auxiliary request and the court granted an injunction prohibiting Meril from supplying a rival valve product in the UPC contracting member states in which the patent is in force. However, the injunction does not apply to all versions of Meril’s valve.
As Meril is the only manufacturer of an extra large version of the type of valve at issue, the Paris court considered there would be a public interest in exempting those versions of the product from the scope of the injunction. This is because it acknowledged that some patients who suffer from aortic stenosis – where the aortic valve into the heart is narrowed – and who would need an extra large valve to be implanted to address the condition could be left without an adequate treatment if those products were included within the scope of the injunction. The court reiterated that to rely on such a public interest defence, “it is essential to demonstrate that [it] is the sole available treatment method or that it represents an improvement upon a known treatment method, resulting in a notable enhancement in patient care”. The exemption in the present case only extends until such time as another equivalent treatment becomes available.
Sarah Taylor of Pinsent Masons, an expert in life sciences patent litigation, said the case provides lessons for businesses on both sides of a patent dispute.
“Injunctions remain an important remedy in a finding of infringement, but they may, in some circumstances, be limited for reasons of public interest,” Taylor said. “Businesses defending against an injunction should take the opportunity to narrow the reach of injunctions where there may be a real public interest reason for doing so. Those seeking injunctions should be aware that such relief may be limited where no alternative treatment method is available.”
The proceedings before the Paris court were initiated by Meril, as part of a long-running dispute between the parties. It asked the court to revoke Edwards’ patent, claiming that it was invalid for added matter, lack of novelty and lack of inventive step. Edwards filed an application to amend the patent and a counterclaim for infringement. After the parties had filed their written pleadings, Edwards requested, and was granted, leave to amend its counterclaim to explicitly extend it to cover Meril’s new heart valve product and delivery system. The court ultimately rejected Meril’s revocation action, maintained the patent as amended by an auxiliary request and upheld Edwards’ counterclaim for infringement.
In assessing the patent’s validity, the Paris court endorsed case law that the Munich Central Division developed last year on the question of inventive step.
Patents can only be obtained for inventions if those inventions are, among other things, new, not obvious, and have an industrial application. To pass the obviousness test, applicants must be able to show that their new product or process delivers an inventive step – i.e. there is a substantive technical advance that someone skilled in the relevant area would not be able to work out easily using their own know-how and information available to them.
Last year, in a case between Amgen on the one hand and Sanofi and Regeneron on the other, the Munich Central Division said that the question of whether there has been an inventive step taken should be assessed holistically.
The first step, it said, is to determine a “realistic starting point” in the prior art that would have been of interest to the skilled person considering a similar “underlying problem” as that which the patent claimed to solve. It said this ‘realistic starting point’ may be one of several and does not need to be the most promising starting point.
The next stage, the Munich court determined, is to ask whether it would be obvious for the skilled person to arrive at the claimed solution in the patent from the ”realistic starting point”. If the skilled person would be motivated to consider and implement the claimed solution in the patent as a next step, then the patent is obvious.
Taylor said the Paris court took the same approach in the Meril v Edwards case, confirming the need for a ”holistic” assessment, and highlighted guidance the court provided on what could constitute a ”realistic starting point” when assessing inventive step.
The court said: “A realistic starting point is a document ‘of interest’ for solving the objective problem. In this regard, it may be assumed that realistic starting points, in general, are the pieces of evidence which disclose the main relevant features as those of the challenged patent and for that reason have constituted the basis for the developing of the inventive idea and/or which address the same or a similar underlying problem.”
Taylor said, however, that the ”holistic approach” backed by the UPC in this case differs from the “problem-solution approach” used by the European Patent Office (EPO) to assess the question of inventive step, which requires the identification of the closest piece of prior art. It had been expected that the UPC would adopt this strict problem-solution approach, and while some divisions of the court have, others, including the Paris Central Division in this case, have deployed the holistic approach. UPC Court of Appeal clarification will ultimately be required to determine binding UPC case law on the issue, but Taylor noted that this difference could have “some implications for identifying the relevant starting point for solving the underlying problem and potentially any experts the parties may want to instruct”.
Taylor said, though, that the different approaches did not lead to a different outcome in the context of the dispute between Meril and Edwards, noting that Meril had lodged parallel opposition proceedings before the EPO before which its arguments pertaining to inventive step were also unsuccessful.
In the context of the parallel EPO proceedings, Taylor said it was noteworthy that the Paris court had exercised its discretion not to ‘stay’ its proceedings – that being, wait for the EPO proceedings to conclude before deciding the dispute before it.
“Clients with proceedings at the EPO and UPC should bear in mind the UPC’s discretion to stay/not stay proceedings,” Taylor said. “The bar to granting a stay is high, with the court keen to meet its aim of reaching a final decision within 12 to 14 months of proceedings being issued and willing to decide matters independently.”
In the present case, in exercising its discretion against a stay in this case, the Paris court considered the fact that neither party had requested a stay, which the court took as implicitly demonstrating the parties’ interest in a prompt decision by the court. Furthermore, at the time the Paris court was informed of the pending opposition proceedings before the EPO, the UPC proceedings were already at an advanced stage – the written phase had concluded, the interim conference had been held, and the oral hearing was imminent. The Paris court also observed that any stay of the proceedings would have the effect of postponing a decision in the infringement action, thereby frustrating Edwards’ interest in a swift protection of its exclusive right.
Taylor highlighted that it took Edwards less than a year from the point it extended its infringement counterclaim to obtaining an injunction against Meril from the Paris court, an injunction which also covers Meril’s new valve product. She said the speed at which the UPC can operate to grant patent rights holders such relief, and the front-loaded nature of UPC proceedings, is something businesses need to factor into their litigation and commercial strategy and planning.
“Parties should collate relevant information as early as possible to ensure written pleadings can be prepared and submitted in the short time frame,” Taylor said. “Additional evidence and information should be considered, collected and prepared early if a public interest defence may be brought into play. Thought should also be given to the approach the UPC may take to assessing inventive step, especially if there are also parallel opposition proceedings at the EPO – the identification of a ‘realistic starting point’ for solving the objective problem is important for the UPC, although it is debateable whether the two ‘different’ approaches are all that inconsistent.”
The European patent at issue in this case is part of a wider family of patents in the longstanding dispute between Edwards and Meril in relation to heart valves. Edwards was successful in pursuing infringement claims against Meril in relation to another of its patents relating to some of Meril’s heart valve products before the Munich Local Division of the UPC last year.

Pakistan’s interior minister Mohsin Naqvi said Thursday that Afghan nationals were behind the two deadly suicide bombings this week, including one in the heart of Islamabad. Naqvi’s claim is likely to further strain the already tense…

Pakistan has deployed its army and…