UBI Research says that sales of foldable OLED panels has been week since the beginning of 2025, as demand for foldable smartphones has been low this year. UBI expects 21.3 million foldable OLED panels to ship in 2025 in total, 14.4% lower than…
Author: admin
-

India police investigate final moments of vehicle in fatal Delhi blast | India
Police investigating the car explosion outside Delhi’s historic Red Fort that killed eight people are focusing on the final movements of the vehicle involved.
The explosion on Monday occurred at about 7pm, a peak time when Delhi’s old city is…
Continue Reading
-
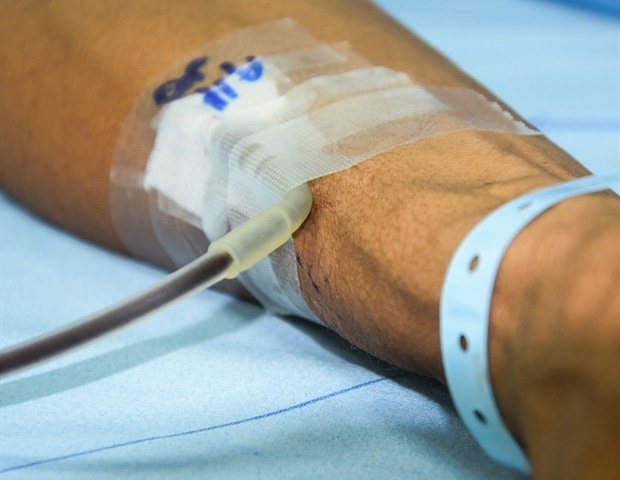
Earlier blood transfusion reduces heart complications post-surgery in high-risk patients
An earlier blood transfusion- done when hemoglobin levels were higher – after major general or vascular surgery among people with heart disease was associated with a lower risk of some complications but not the most severe ones,…
Continue Reading
-

KP Govt imposes complete ban on teachers’ transfers
– Advertisement –
PESHAWAR, Nov 11 (APP):The Khyber Pakhtunkhwa (KP) government has imposed a complete ban on all postings and transfers of male and female teaching staff in the Elementary and Secondary Education Department.
The decision was made…
Continue Reading
-

New data supports earlier use of evolocumab in high-risk diabetes
Adding the PCSK9 inhibitor evolocumab to a high-intensity, cholesterol-lowering regimen reduced the risk of a first major cardiovascular event among adults with atherosclerotic cardiovascular disease (ASCVD) or diabetes , according to a preliminary late-breaking science presentation today at the American Heart Association’s Scientific Sessions 2025. The meeting, Nov. 7-10, in New Orleans, is a premier global exchange of the latest scientific advancements, research and evidence-based clinical practice updates in cardiovascular science.
“The results from the VESALIUS-CV trial represent the first demonstration of improved cardiovascular outcomes with a PCSK9 inhibitor, or any non-statin for that matter, in patients without a previous heart attack or stroke who are already being treated with a high-intensity lipid-lowering regimen ,” said lead study author Erin A. Bohula, M.D., D.Phil., an assistant professor of medicine at Harvard Medical School, Brigham & Women’s Hospital and an investigator with the TIMI Study Group.
According to the American Heart Association, atherosclerotic cardiovascular disease, otherwise known as ASCVD, is caused by plaque buildup in arterial walls and refers to conditions that include:
- Coronary Heart Disease (CHD), such as myocardial infarction, angina and coronary artery stenosis.
- Cerebrovascular disease, such as a transient ischemic attack, ischemic stroke and carotid artery stenosis.
- Peripheral artery disease such as claudication.
- Aortic atherosclerotic disease, such as abdominal aortic aneurysm and descending thoracic aneurysm.
Currently, ASCVD-related conditions remain the leading cause of morbidity and mortality globally.
The VESALIUS-CV trial examined if adding evolocumab, a non-statin PCSK9 inhibitor medication to lower low-density lipoprotein cholesterol (LDL-C), to existing cholesterol treatment reduced the risk of a first major cardiovascular event in people with ASCVD or diabetes who had no history of a major CV event, such as heart attack or stroke.
After an average of 4.6 years of follow-up, the study found:
- Patients in the evolocumab group had a significantly reduced risk of the dual primary endpoints: by 25% for coronary heart disease deaths, heart attack or ischemic stroke and by 19% for coronary heart disease death, heart attack, ischemic stroke or ischemia-driven arterial revascularization, compared to patients in the placebo group.
- There was also a 27% reduction in cardiovascular death, heart attack or ischemic stroke and a 36% reduction in heart attack among participants in the evolocumab group, compared to placebo.
- Nominally lower rates of death from cardiovascular causes (2.8% versus 3.6%, respectively) and death from all causes (7.9% versus 9.7%, respectively) were noted in the evolocumab group compared to the placebo group.
- Findings for the dual primary endpoints were consistent across key subgroups, including in participants with high-risk diabetes without qualifying ASCVD, which represented one-third of the total study population.
- In a sub-study that evaluated participants’ lipids measures over time, the median LDL-C at enrollment was 115 mg/dL. At 48 weeks, LDL-C was lowered by nearly 55% in the evolocumab group, resulting in a median LDL-C level of 45 mg/dL.
- In contrast, LDL-C levels remained elevated among those in the placebo group, at a median of 109 mg/dL.
“Interestingly, the magnitude of cardiovascular benefit per unit of LDL-C reduction is similar to what has been observed in statin trials, as described by the Cholesterol Treatment Trialists’ Collaboration,” said Bohula.
We suspect this is related to the longer follow up in our study, as compared to prior, shorter PCSK9 inhibitor trials, that may have underestimated the long-term clinical benefit. It has been well-described that there is a delay in the onset for cardiovascular benefits from lowering LDL-C levels, and it takes time for these benefits to be measurable.”
Erin A. Bohula, Assistant Professor, Medicine, Brigham & Women’s Hospital
Study details, background and design:
- The study included 12,257 adults who were an average age of 66 years old.
- Of the participants, 43% were women, and the majority (93%) of participants self-identified as white, and about 17% self-reported their race as Hispanic or Latino.
- The study was conducted at 745 health care sites in 33 countries including the U.S. between June 2019 and November 2021.
- Participants were eligible for the study if they had an LDL-C of at least 90 mg/dL (or met non-high-density lipoprotein cholesterol or apolipoprotein B criteria), met study inclusion criteria for atherosclerosis (coronary artery, peripheral artery or cerebrovascular disease) or high-risk diabetes, and had at least one other cardiovascular risk factor. Patients with a prior heart attack or stroke were excluded.
- At the start of the study, about two-thirds of the participants met the study inclusion criteria for atherosclerosis (without a prior heart attack or stroke), and 50% met inclusion criteria for diabetes; the average level LDL-C level was 122 mg/dL; the average level of apolipoprotein-B (Apo-B) was 101 mg/dL; and 72% of participants were on a high-intensity lipid-lowering regimen.
- Participants were randomized to one of the two treatment groups: 140 mg of evolocumab injected under the skin every two weeks, or a placebo also injected every 2 weeks, for the duration of the trial, an average of 4.6 years.
The study had several limitations to note. There was a small group of patients (8%) who were not being treated with any cholesterol-lowering treatment at the beginning of the study. The authors noted that the majority of patients (72%) were on a high-intensity regimen when they enrolled in the trial. In addition, the researchers suggest future studies including adults from various racial and ethnic backgrounds are needed to confirm if these findings apply across diverse populations.
“Together with data from genetic studies of PCSK9 variants and other PCSK9 inhibitor outcomes studies, our findings suggest that long-term lowering with PCSK9 inhibitors can help to improve cardiovascular morbidity and potentially mortality over time. The findings also support the use of intensive LDL-C lowering to achieve targets of around 40 mg/dL to help prevent a first major cardiovascular event ,” said Bohula.
Evolocumab is a newer type of cholesterol-lowering medication called a PCSK9 inhibitor that binds to and inactivates a protein in the liver to lower LDL cholesterol. Evolocumab is FDA-approved to treat high LDL-C levels; however, PCSK9 inhibitors may not be covered by some health insurance plans, which may be a barrier for some people. Other research studies have confirmed that evolocumab reduces the risk of major adverse cardiovascular events in people who have ASCVD, peripheral artery disease (PAD) with symptoms or have had a prior heart attack or stroke.
Source:
American Heart Association
Continue Reading
-

Field Marshal Asim Munir attends 45th PARA Meet and MCJ Centenary
The Closing Ceremony of the 45th Pakistan Army Rifle Association (PARA) Central Meet was held at the Army Marksmanship Unit (AMU), Jhelum, with Field Marshal Syed Asim Munir, NI (M), HJ, Chief of Army Staff (COAS), attending as the Chief Guest.
…
Continue Reading
-

Hema Malini reacts to Dharmendra death reports, calls it ‘unforgivable and disrespectul’
Hema Malini and Esha Deol refute media speculation on Dharmendra`s health, confirming he is recovering and urging privacy.
Hema Malini, Dharmendra | Photo: PTI
Mumbai: In the wake of growing speculations…
Continue Reading
-
Infosys Positioned as a Leader in the 2025 Gartner® Magic Quadrant™ for Public Cloud IT Transformation Services for the Third Consecutive Year
Infosys (NSE, BSE, NYSE: INFY), a global leader in next-generation digital services and consulting, today announced that it has been positioned as a Leader in the 2025 Gartner® Magic Quadrant™ for Public Cloud IT Transformation Services (PCITS), for the third year in a row.
This recognition reflects Infosys’ consistent excellence in delivering transformational outcomes through cloud-native professional and managed services. It is also a testament of Infosys’ ability to execute and completeness of vision, underscoring its strategic investments in cloud innovation and enterprise automation. Infosys Cobalt continues to accelerate enterprise cloud journeys with a strong focus on zero-touch operations, automated healing, and AIOps-driven resiliency.
Anant Adya, EVP and Service Offering Head, Infosys, said, “Being recognized as a Leader in the 2025 Gartner Magic Quadrant for Public Cloud IT Transformation Services for the third consecutive year strongly reaffirms the trust our clients place in Infosys. With Infosys Cobalt, we are not just guiding our clients through cloud, data, and Enterprise AI transformation – we are co-innovating with them to build the digital businesses of the future.”
Gartner Disclaimer
Gartner, Magic Quadrant for Public Cloud IT Transformation Services, 4 August 2025.
GARTNER is a registered trademark and service mark and MAGIC QUADRANT is a trademark of Gartner, Inc. and/or its affiliates in the U.S. and internationally and are used herein with permission. All rights reserved. Gartner does not endorse any vendor, product or service depicted in its research publications, and does not advise technology users to select only those vendors with the highest ratings or other designation. Gartner research publications consist of the opinions of Gartner’s research organization and should not be construed as statements of fact. Gartner disclaims all warranties, expressed or implied, with respect to this research, including any warranties of merchantability or fitness for a particular purpose.For more information, please visit: https://infosys.com/cobalt and https://infosys.com/topaz
About Infosys
Infosys is a global leader in next-generation digital services and consulting. Over 320,000 of our people work to amplify human potential and create the next opportunity for people, businesses, and communities. We enable clients in more than 59 countries to navigate their digital transformation. With over four decades of experience in managing the systems and workings of global enterprises, we expertly steer clients, as they navigate their digital transformation powered by cloud and AI. We enable them with an AI-first core, empower the business with agile digital at scale and drive continuous improvement with always-on learning through the transfer of digital skills, expertise, and ideas from our innovation ecosystem. We are deeply committed to being a well-governed, environmentally sustainable organization where diverse talent thrives in an inclusive workplace.
Visit www.infosys.com to see how Infosys (NSE, BSE, NYSE: INFY) can help your enterprise navigate your next.
Safe Harbor
Certain statements in this release concerning our future growth prospects, or our future financial or operating performance, are forward-looking statements intended to qualify for the ‘safe harbor’ under the Private Securities Litigation Reform Act of 1995, which involve a number of risks and uncertainties that could cause actual results or outcomes to differ materially from those in such forward-looking statements. The risks and uncertainties relating to these statements include, but are not limited to, risks and uncertainties regarding the execution of our business strategy, increased competition for talent, our ability to attract and retain personnel, increase in wages, investments to reskill our employees, our ability to effectively implement a hybrid work model, economic uncertainties and geo-political situations, technological disruptions and innovations such as artificial intelligence (“AI”), generative AI, the complex and evolving regulatory landscape including immigration regulation changes, our ESG vision, our capital allocation policy and expectations concerning our market position, future operations, margins, profitability, liquidity, capital resources, our corporate actions including acquisitions, and cybersecurity matters. Important factors that may cause actual results or outcomes to differ from those implied by the forward-looking statements are discussed in more detail in our US Securities and Exchange Commission filings including our Annual Report on Form 20-F for the fiscal year ended March 31, 2025. These filings are available at www.sec.gov. Infosys may, from time to time, make additional written and oral forward-looking statements, including statements contained in the Company’s filings with the Securities and Exchange Commission and our reports to shareholders. The Company does not undertake to update any forward-looking statements that may be made from time to time by or on behalf of the Company unless it is required by law.
Media contact
For more information, please contact: PR_Global@infosys.com
Continue Reading
-

Why Kay Barron Left Net-a-Porter for the World of Live Shopping
Vogue: Why do you think luxury has been slow to experiment with video shopping?
I think because often it’s people in their houses, it’s scrappy, so brands look at that and think, “Oh god, that’s not for us.” But there are so many…
Continue Reading
-

Maximizing the Seestar S50 for the Moon, Jupiter, and Saturn » World Business Outlook
The ZWO Seestar S50 has revolutionized amateur astrophotography, making deep-sky objects accessible with push-button ease. While its short focal length of 250mm makes it a deep-sky darling, the temptation to turn this smart scope toward the…
Continue Reading