Biomaterial vaccines using pathogen-specific antigens could significantly lower patients’ risk of infection from implanted medical devices
By Benjamin Boettner
By Benjamin Boettner
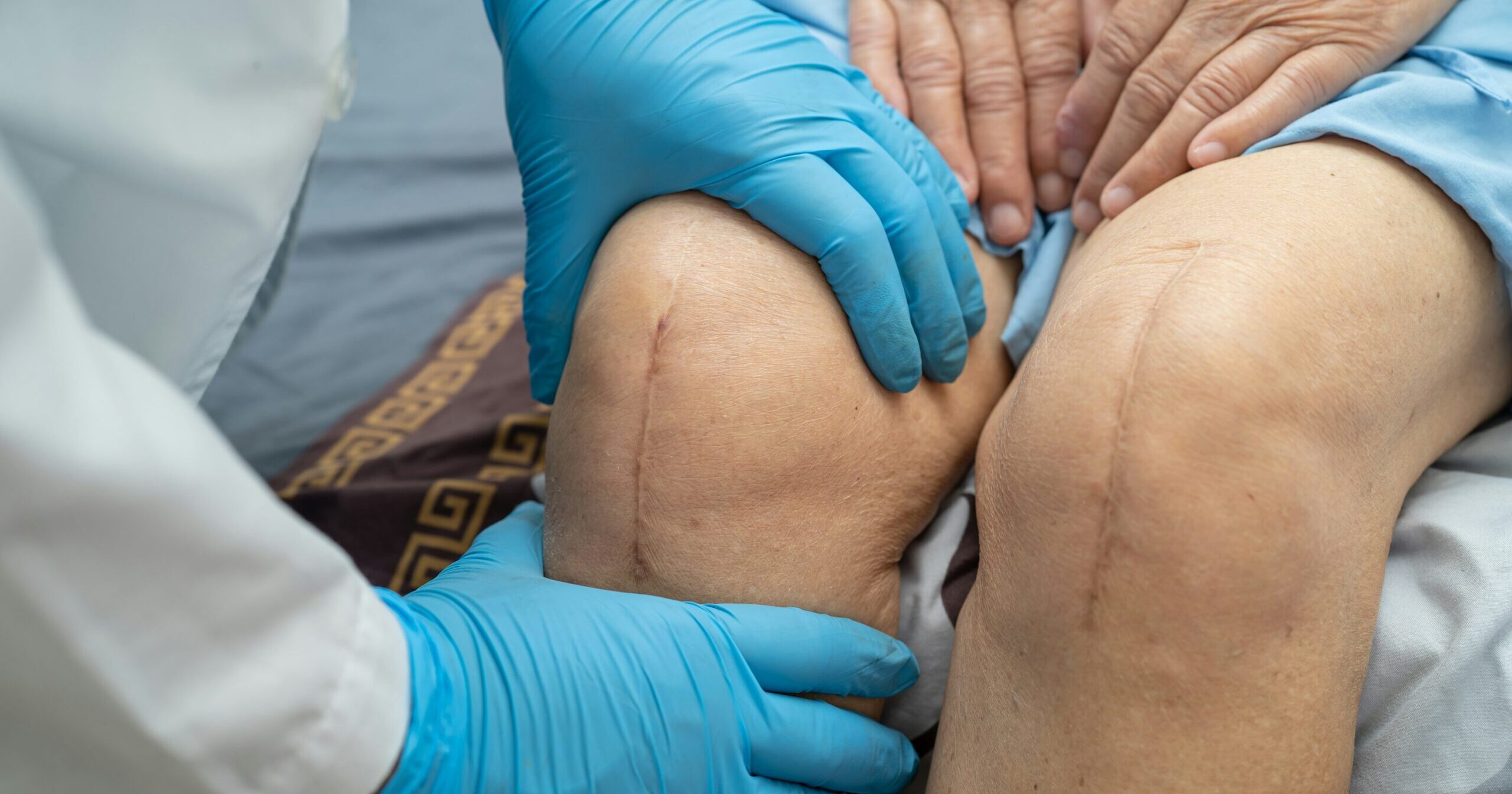
Patients with implanted medical devices like orthopedic joint replacements, pacemakers, and artificial heart valves run a small but significant risk…

A new three-pronged blood test can highlight people with a nearly tripled risk for heart attack, Wake Forest University researchers said in a new study. File Photo by Tamas Soki/EPA
A new three-pronged blood test can highlight people with a…

Combination therapy with cadonilimab, a first-in-class bispecific antibody targeting both PD-1 and CTLA-4, and chemotherapy offers encouraging antitumor activity and manageable safety as a first-line treatment for patients with advanced esophageal squamous cell carcinoma (ESCC), according to findings from a phase 2 study (NCT05522894).1
The trial successfully met its primary end point, achieving an objective response rate (ORR) of 81.4% (95% CI, 66.6%–91.6%) and a disease control rate (DCR) of 97.7% (95% CI, 86.2%–99.9%) The median progression-free survival (PFS) was 7.10 months (95% CI, 5.68–8.48), with overall survival (OS) data still immature.
The authors note that the ORR of 81.4% is numerically superior to the 69.3%–72.1% rates reported in phase 3 trials of PD-1 inhibitors plus chemotherapy (ESCORT-1st2 and JUPITER 063), though they caution that cross-trial comparisons should be interpreted with care due to differences in patient populations.1
Notably, the treatment showed significant efficacy across all patient subgroups, irrespective of PD-L1 expression levels. Patients with a low PD-L1 combined positive score (CPS) of < 1 achieved an ORR of 87.5% (95% CI, 47.9%–99.7%) and a median PFS of 8.41 months, suggesting the combination could be a potent new option for a difficult-to-treat population.
The trial was designed to address the unmet need for more effective first-line treatments for advanced ESCC, a malignancy with a poor prognosis. While the current standard of care—a PD-1/PD-L1 inhibitor combined with chemotherapy—has improved outcomes, a significant portion of patients do not achieve durable responses.
“The blockade of both PD-1 and CTLA-4 strengthens the tumor immune response and achieves an effect more than merely additive,” study authors wrote.
The study was an open-label, single-arm, multicenter phase 2 study conducted in 3 hospitals in China. The primary end point was ORR, with PFS, DCR, OS, and safety as secondary end points.
A total of 43 patients were enrolled between February 2023 and April 2024. All patients had an ECOG performance status of 0 or 1 and had not received prior systemic treatment for advanced disease.
The treatment regimen consisted of a combination phase of 10 mg/kg cadonilimab administered intravenously (IV) on day 1 of a 21-day cycle and paclitaxel or nab-paclitaxel and cisplatin on day 1. This was continued for up to 6 cycles. Following this phase, patients continued with cadonilimab monotherapy every 21 days until disease progression, unacceptable toxicity, or a maximum of 24 months.
The safety profile of cadonilimab plus chemotherapy was considered manageable, with no new or unexpected safety signals observed. All 43 patients (100%) experienced a treatment-related adverse event (TRAE) of any grade; 53.5% (n = 23) of patients experienced a grade 3 or 4 TRAE. No grade 5 TRAEs occurred.
All-grade immune-related AEs (IRAEs) were observed in 19 patients (44.2%). Grade 3 to 4 IRAEs occurred in 5 patients (11.6%).
TRAEs led to the discontinuation of cadonilimab in 7 patients (16.3%).
The study conducted an exploratory analysis of cell-free DNA (cfDNA) methylation as a potential biomarker for treatment response, yielding highly significant results.
A unique 5-gene methylation panel (APBA2, EPAS1, TRIM58, ITPKA, LINC00554) was identified that could stratify patients into response groups. The average methylation level of this 5-CpG signature before treatment robustly predicted outcomes. A higher baseline methylation level (hypermethylation) was significantly associated with nonresponders (P <.001).
Using a 35% methylation level as a threshold, patients with < 35% baseline methylation had a 100% response rate (19 of 19 achieved partial response). Patients with >35% baseline methylation had only a 35.3% response rate (6 of 17 responded).
Posttreatment analysis revealed that responders tended to have stabilized methylation levels, while non-responders exhibited global hypomethylation. This suggests that cfDNA methylation could serve not only as a static predictive biomarker but also as a dynamic tool for real-time monitoring of tumor-immune interactions.
This request seems a bit unusual, so we need to confirm that you’re human. Please press and hold the button until it turns completely green. Thank you for your cooperation!

Heads up stargazers! The Southern Taurid meteor shower peaks overnight on Nov. 4-5, when a flurry of bright meteors could potentially be seen streaking through Earth’s sky as our planet passes through the outer edge of a debris swarm shed by the…

Sustainable biomanufactured textiles are produced by Tandem Repeat Technologies.
PHILADELPHIA, Nov. 3, 2025 /PRNewswire-PRWeb/ — Tandem Repeat Technologies, Inc. (Tandem), a leader in advanced protein fiber biomanufacturing, today…