Genetic tweaks changed how the hip bones of early humans developed, which allowed them to start walking upright on two legs, according to new research. Photo by Adobe Stock/HealthDay News
Two small changes in human DNA may have played a big role…
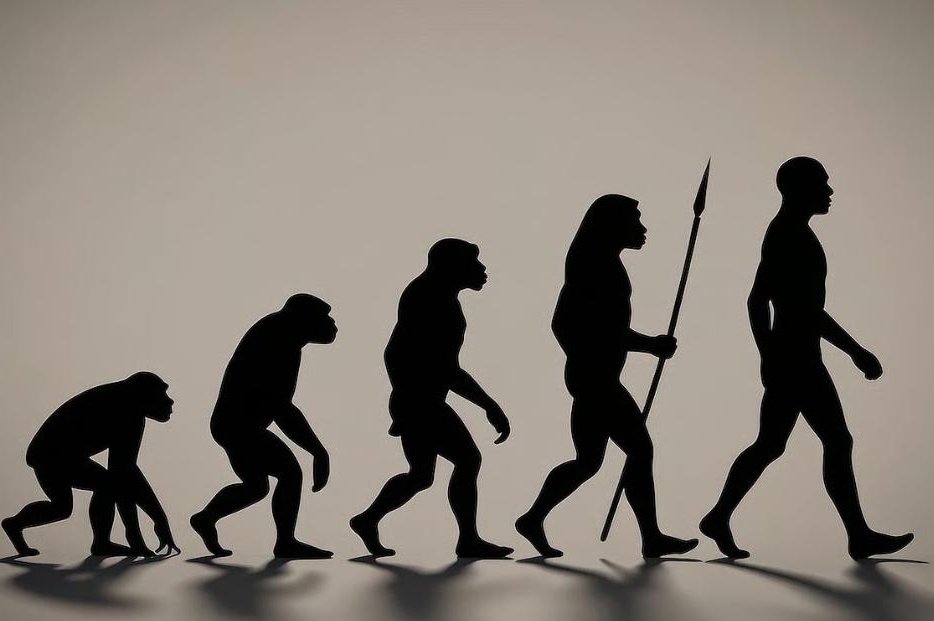
Genetic tweaks changed how the hip bones of early humans developed, which allowed them to start walking upright on two legs, according to new research. Photo by Adobe Stock/HealthDay News
Two small changes in human DNA may have played a big role…

Rihanna is a true bag obsessive. Tom Ford-era Gucci bags in iconic python, ultra-rare Louis Vuitton pochettes, and Dior saddle bags. Bowling, doctor’s, east-wests, baguettes: Rihanna is stocked up. What’s in any of those bags? Who’s to say.
Continue Reading

Pharmacy Times: What role can pharmacists play in identifying and counseling patients who may benefit from switching to low-sodium oxybate?

October 2025 proved to be a significant month for the oncology landscape, marked by a wave of key regulatory decisions from the US FDA. This period saw important full and accelerated drug approvals across various cancer types, expanding treatment options for patients with both solid tumors and hematologic malignancies.
From new combination maintenance therapies for extensive-stage small cell lung cancer (ES-SCLC) and an adjuvant treatment for high-risk cutaneous squamous cell carcinoma (CSCC), to a crucial approval in relapsed or refractory acute myeloid leukemia (AML) with a specific mutation, the month delivered on several anticipated action dates.
Furthermore, the FDA continued to expedite the development of promising agents by granting several breakthrough therapy and fast track designations, signaling a robust pipeline of innovative oncology therapeutics on the horizon. This roundup delves into the most notable FDA news and designations that will shape cancer care moving forward.
On October 1, the FDA accepted the supplemental biologics license application (sBLA) for trastuzumab deruxtecan (T-DXd; Enhertu) followed by paclitaxel (Taxol), trastuzumab (Herceptin), and pertuzumab (Perjeta; THP) for the neoadjuvant treatment of adult patients with HER2-positive stage 2 or 3 breast cancer. A Prescription Drug User Fee Act (PDUFA) target action date of May 18, 2026, has been set.
Also on October 1, the FDA granted fast track designation to ETX-636, a pan mutant-specific allosteric PIK3CA inhibitor, for the treatment of patients with PIK3CA-mutant, HR-positive, HER2-negative advanced breast cancer.
On October 2, the FDA approved the combination of lurbinectedin (Zepzelca) and atezolizumab (Tecentriq) or atezolizumab and hyaluronidase-tqjs (Tecentriq Hybrezas) as a first-line maintenance treatment in patients with ES-SCLC whose disease has not progressed following first-line induction therapy with atezolizumab, carboplatin, and etoposide.
Also on October 2, the FDA granted orphan drug designation to AMXT 1501 in combination with difluoromethylornithine (Iwilfin; DFMO) for the treatment of patients with neuroblastoma.
On October 6, the FDA accepted for priority review the biologics license application of Orca-T, an investigational allogeneic T-cell immunotherapy, for the treatment of hematologic malignancies including acute myeloid leukemia, acute lymphoblastic leukemia, and myelodysplastic syndromes. The FDA has set a PDUFA target action date of April 6, 2026.
On October 8, the FDA issued a complete response letter to the new drug application of Daysnoc, a lower-dose, bioequivalent formulation of dasatinib (Sprycel), based on good manufacturing practice issues observed at Xspray’s contract manufacturer.
Also on October 8, the FDA approved cemiplimab (Libtayo) as adjuvant treatment in adults with high-risk CSCC.
The FDA granted fast track designation to the investigational therapeutic agent WTX-124 for the potential treatment of patients with locally advanced or metastatic cutaneous melanoma that has progressed following standard-of-care immunotherapy, also on October 8.
On October 9, the FDA granted fast track designation to the antibody-drug conjugate (ADC) ADCE-D01 for the treatment of soft tissue sarcoma.
On October 13, the FDA granted breakthrough therapy designation to the bifunctional antibody ficerafusp alfa (BCA101) in combination with pembrolizumab (Keytruda) for the first-line treatment of HPV-negative, recurrent or metastatic head and neck squamous cell carcinoma.
On October 14, the FDA granted breakthrough therapy designation to the investigational BCL-2 inhibitor sonrotoclax (BGB-11417) for the treatment of adult patients with relapsed or refractory mantle cell lymphoma following therapy with a BTK inhibitor and an anti-CD20 agent.
Also on October 14, NG-350A, an intravenously delivered oncolytic immunotherapy, was granted fast track designation by the FDA for the treatment of mismatch repair-proficient (pMMR) locally advanced rectal cancer.
On October 15, the FDA granted orphan drug designation to the investigational mitochondrial cell therapy MNV-201 for the treatment of myelodysplastic syndrome.
On October 16, the FDA granted fast track designation to the novel immunotherapy EO2463 for the treatment of follicular lymphoma, backed by positive interim data from the ongoing phase 2 SIDNEY trial (NCT04669171).
On October 21, the FDA accepted the new drug application for XS003, a formulation referencing the TKI nilotinib (Tasigna), for treatment of chronic myeloid leukemia. A PDUFA target action date has been set for June 18, 2026.
Also on October 21, the FDA granted priority review to the sBLA of enfortumab vedotin-ejfv (Padcev) in combination with pembrolizumab, radical cystectomy, and standard pelvic lymph node dissection as perioperative treatment for patients with muscle-invasive bladder cancer who are ineligible for or decline cisplatin-containing chemotherapy.
On October 22, the FDA gave fast track designation to MT-125, a first-in-class dual small-molecule inhibitor, for the treatment of glioblastoma.
On October 23, the FDA granted fast track designation to the novel amanitin-based ADC pamlectabart tismanitin (HDP-101) for treatment of patients with relapsed or refractory multiple myeloma.
On October 24, the FDA approved the application for revumenib (Revuforj) in the treatment of relapsed or refractory NPM1-mutant AML, supported by positive data from the phase 2 portion of the AUGMENT-101 trial (NCT04065399).
Also on October 24, the FDA granted breakthrough therapy designation to zenocutuzumab (Bizengri) for the treatment of patients with advanced unresectable or metastatic cholangiocarcinoma harboring an NRG1 gene fusion. This designation was supported by results from the ongoing phase 2 eNRGy trial (NCT02912949).
On October 27, the FDA granted fast track designation to JSKN003, a biparatopic HER2-targeting ADC, for the treatment of platinum-resistant ovarian cancer, regardless of HER2 expression.
Also on October 27, the oral, multiselective inhibitor daraxonrasib (RMC-6236) was awarded orphan drug designation by the FDA for the treatment of pancreatic cancer.
The FDA granted orphan drug designation to ZEN-3694, a novel oral therapy, for the treatment of NUT carcinoma, also on October 27.

Paul Homchick bought his first fountain pen three decades ago. He was working as an engineering consultant and wanted to seem trustworthy as he took notes.
Since retiring, the 76-year-old has been more interested in exploring different types of nibs, the metal tip of a fountain pen, than impressing clients. To save money, he decided to give Chinese brands a shot.
Copyright ©2025 Dow Jones & Company, Inc. All Rights Reserved. 87990cbe856818d5eddac44c7b1cdeb8

Defending champion Coco Gauff faces fellow American Jessica Pegula in the second match in the Stefanie Graf Group on Sunday after Elena Rybakina and Iga Swiatek triumphed on the opening day.
Sabalenka has qualified for the year-end Finals for the…

Grand Theft Auto (GTA) 6 developer Rockstar has denied accusations of union-busting. The company’s statement comes after the gaming studio reportedly fired over 30 employees across its UK and Canadian offices. Rockstar’s parent company,…

Active Re plans to make Asia-Pacific a cornerstone of its global portfolio within five years, expanding its presence, strengthening broker and client…

The Blackmagic Design Pocket Cinema Camera 4 K remains one of the most admired tools for filmmakers who care about cinematic storytelling on a realistic budget. Its mix of dynamic-range depth, color science, and RAW flexibility has…