Have you ever wondered how you could make life awesome?
From AI-powered[1] productivity and enhanced durability,[2] to massive immersive displays and more, the latest additions to the Galaxy A…

Have you ever wondered how you could make life awesome?
From AI-powered[1] productivity and enhanced durability,[2] to massive immersive displays and more, the latest additions to the Galaxy A…
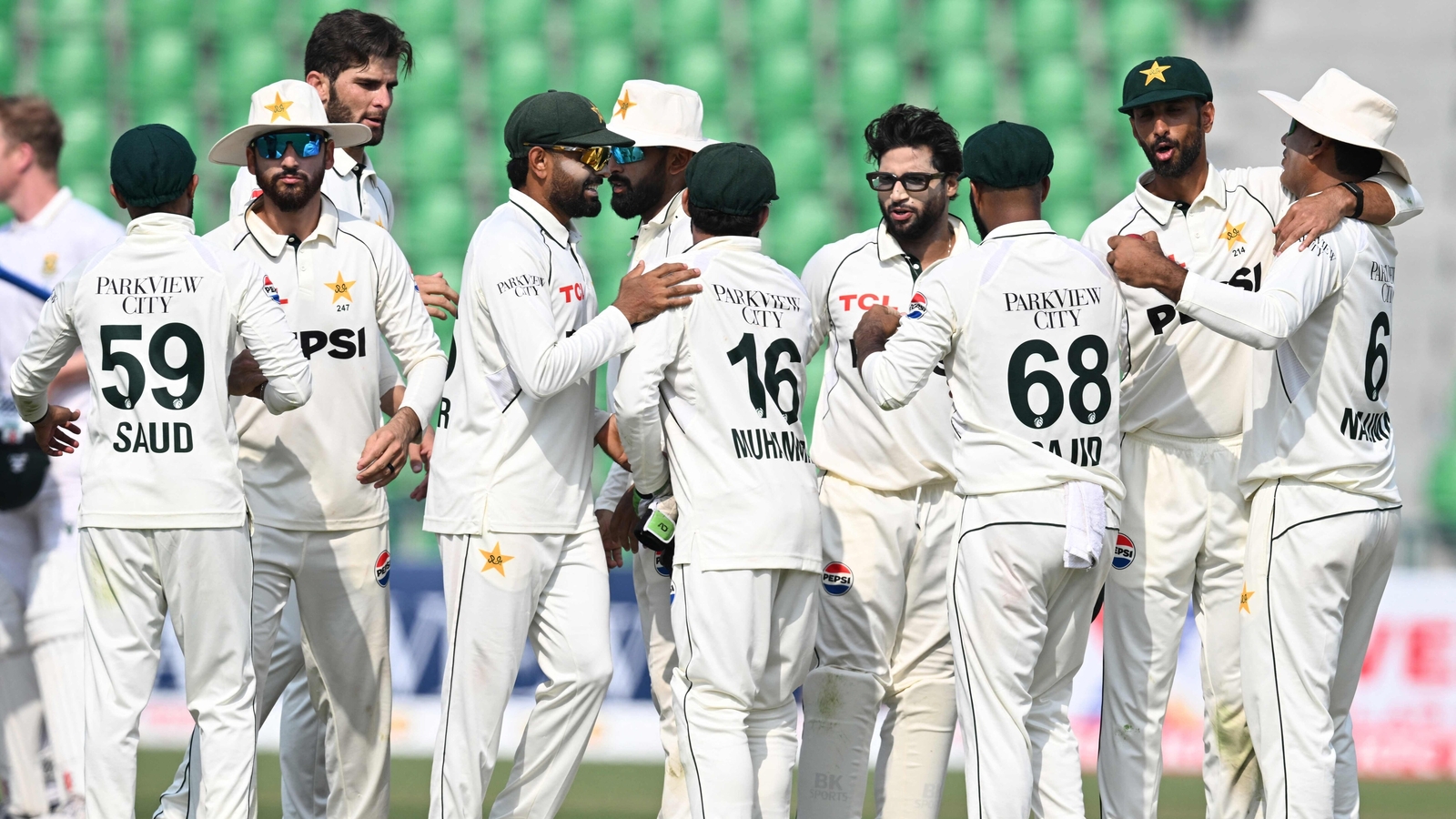
Shan Masood-led Pakistan will look to wrap up the two-match Test series when the side takes the field against South Africa in Rawalpindi. The hosts registered a comprehensive victory in the first Test as Noman Ali and Sajid Khan spun a web…

Every week, we like to take the time to check out all of the top-trending titles on Steam over the last week. This is ranked by their Followers gained throughout, which gives us an idea what’s been…
You don’t have permission to access “http://www.alvarezandmarsal.com/press-release/turnaround-industry-survey-signs-of-recovery-overshadowed-by-persistent-business-struggles” on this server.
Reference #18.8d5e6cc1.1760922791.28c70feb
https://errors.edgesuite.net/18.8d5e6cc1.1760922791.28c70feb

At a Glance
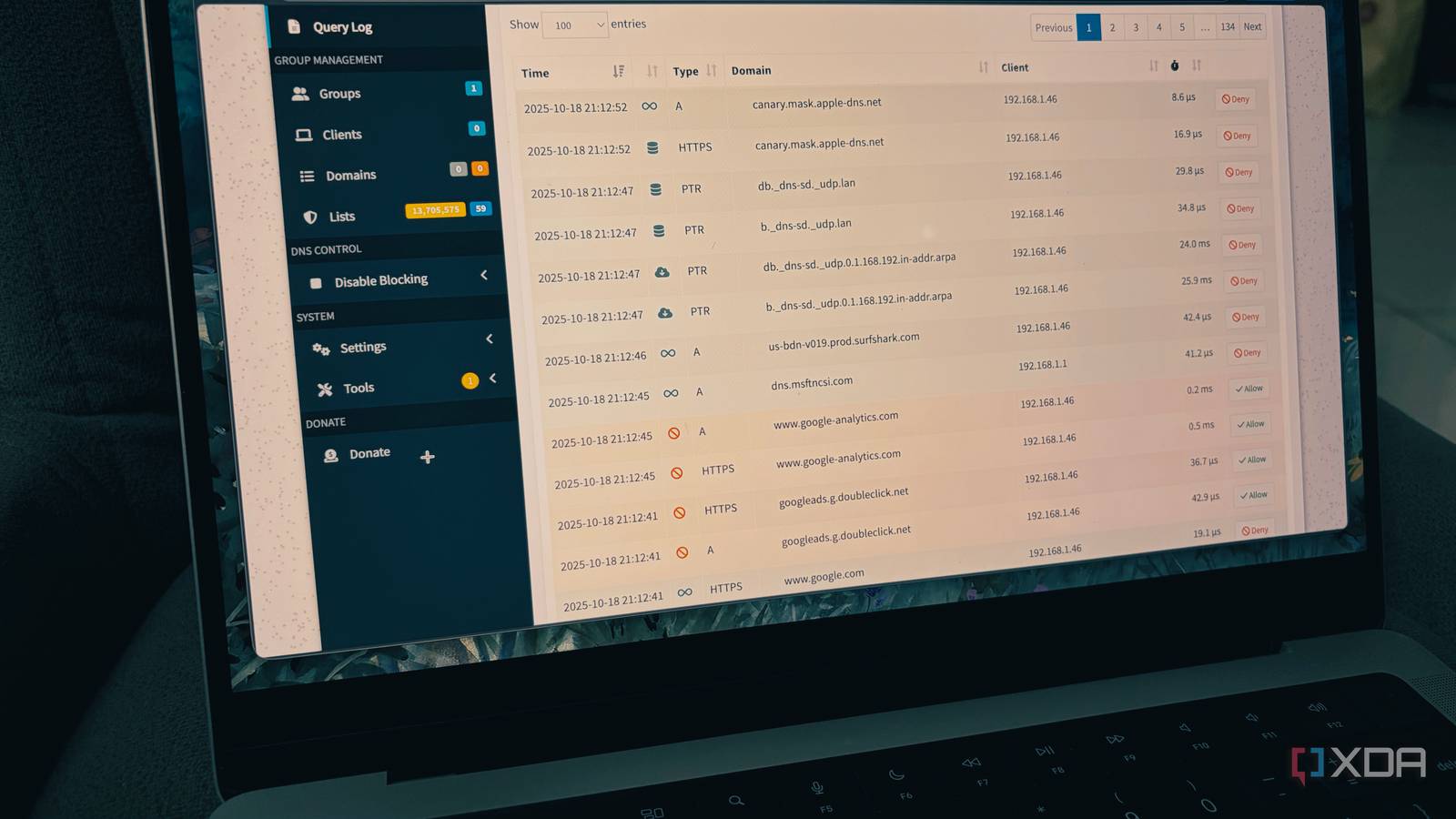
If your home network feels sluggish, unreliable, and constantly bombarded by digital noise, you are not alone. After all, we spend fortunes on fast internet and mesh Wi-Fi, yet our experience is constantly affected by pop-ups, trackers, and…