Introduction
Renal cell carcinoma (RCC) is a common malignancy, originating from the epithelium of the renal tubules and accounting for approximately 80% of malignant tumors of the kidney. It mainly affects middle-aged and elderly patients, and…

Renal cell carcinoma (RCC) is a common malignancy, originating from the epithelium of the renal tubules and accounting for approximately 80% of malignant tumors of the kidney. It mainly affects middle-aged and elderly patients, and…

Chevrolet’s decision to offer Carbon Revolution wheels on the C8 Corvette lineup, especially on the Z06 and E-Ray, is about physics first and looks second. While it’s obvious Chevrolet knows that customers would go crazy over the stylistic…
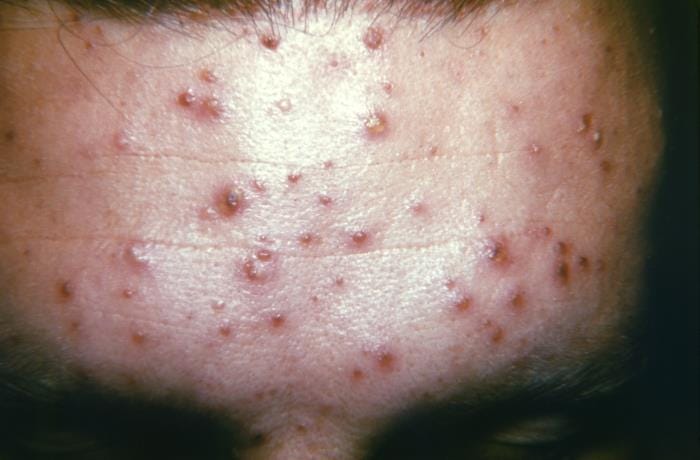
The Prison Officers’ Association (POA) posted the following on Friday:
There is an outbreak at the Maximum Security Prison of Chicken Pox. We advise our members to adhere to the strictest protocols to reduce the risk of transmission to your self…

Minnesota United today announced that Eric Ramsay will join West Bromwich Albion F.C. of the English Championship as their next head coach. MNUFC assistant coach Dennis Lawrence will also depart the club and join…

Ashton was last seen wearing a dark red jacket, a dark backpack, and was carrying a shovel.
If you have any information on the whereabouts of Ashton Calliou, please contact the Grande Prairie RCMP detachment at 780-830-5701. If you wish to remain…
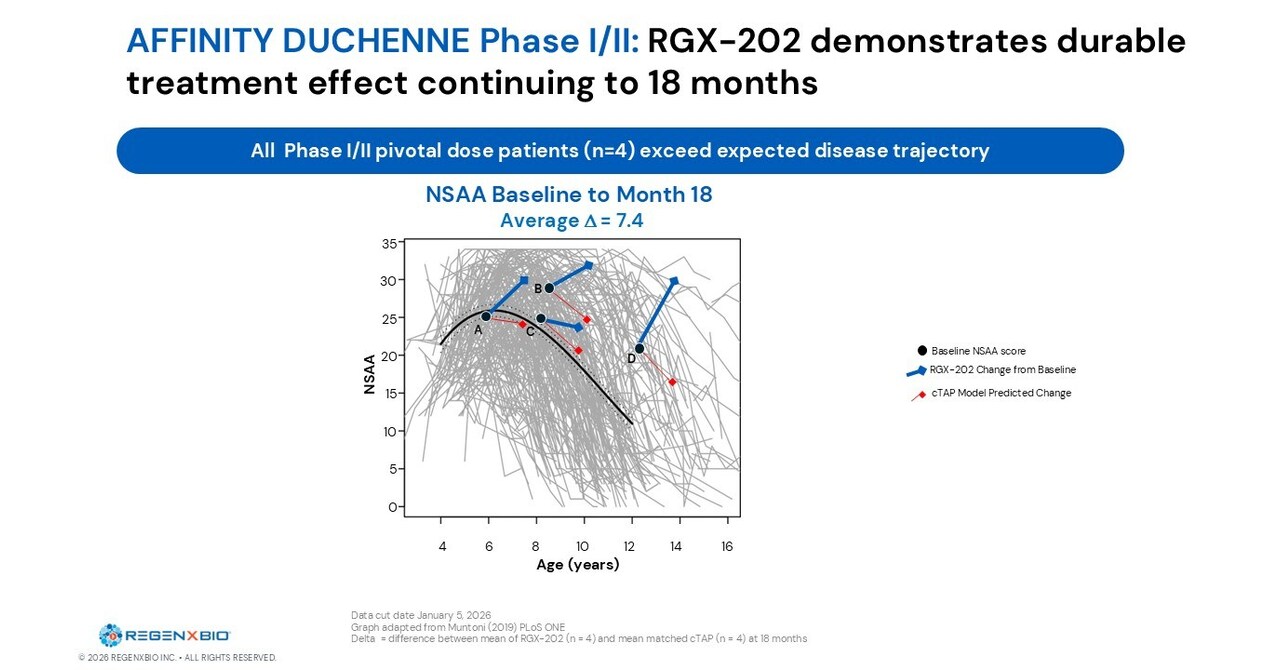
ROCKVILLE, Md., Jan. 11, 2026 /PRNewswire/ — REGENXBIO Inc. (Nasdaq: RGNX) highlighted progress and upcoming anticipated milestones across its pipeline of AAV gene therapies for rare and retinal diseases.
“2026 is set to be a transformative year for REGENXBIO, as we enter commercial stage with two near-term catalysts from our three late-stage assets and a clear path to sustained growth,” said Curran Simpson, President and CEO, REGENXBIO. “We are starting the year with exciting new long-term data for our Duchenne program, demonstrating how our comprehensive strategy to maximize the potential for therapeutic benefit across all our programs is resulting in positive outcomes for patients. We are continuing to set the bar high for how potentially life-changing gene therapies are discovered, developed, and manufactured; this year we are sharply focused on advancing our commercial readiness to enable successful launches of these medicines for patients in need.
CLINICAL PROGRAM UPDATES AND 2026 ANTICIPATED MILESTONES
RGX-202 for Duchenne Muscular Dystrophy
New Functional Data
Clinical Trial and Regulatory Milestones
Clemidsogene lanparvovec (RGX-121) for MPS II, also known as Hunter syndrome
Surabgene lomparvovec (sura-vec, ABBV-RGX-314) for wet age-related macular degeneration (wet AMD) and diabetic retinopathy (DR)
Sura-vec is being developed in collaboration with AbbVie, and could be the first gene therapy for a non-rare disease, if approved.
Leading Gene Therapy Capabilities
REGENXBIO is one of the only gene therapy companies with fully in-house, end-to-end capabilities from capsid engineering and discovery through commercial-ready manufacturing, designed to reliably scale supply and realize the blockbuster potential of its gene therapy portfolio. At the REGENXBIO Manufacturing Innovation Center, in Rockville, Md., REGENXBIO expects to continue to build supply intended for potential commercial launches. Process performance qualification lots have been completed for RGX-202.
REGENXBIO continues to expand the therapeutic potential of AAV gene delivery through capsid discovery and engineering. The Company is approaching IND readiness for the treatment of geographic atrophy using a new capsid that has demonstrated higher transgene expression via suprachoroidal delivery to the eye.
J.P. Morgan Healthcare Conference Presentation
President and CEO Curran Simpson will present at the J.P Morgan Healthcare Conference on Wednesday, January 14, 2026 at 10:30 a.m. PT. A live webcast of the presentation can be accessed in the Investors section of REGENXBIO’s website at www.regenxbio.com. An archived replay of the webcast will be available for approximately 30 days following the presentation.
ABOUT REGENXBIO Inc.
REGENXBIO is a biotechnology company on a mission to improve lives through the curative potential of gene therapy. Since its founding in 2009, REGENXBIO has pioneered the field of AAV gene therapy. REGENXBIO is advancing a late-stage pipeline of one-time treatments for rare and retinal diseases, including RGX-202 for the treatment of Duchenne; clemidsogene lanparvovec (RGX-121) for the treatment of MPS II and RGX-111 for the treatment of MPS I, both in partnership with Nippon Shinyaku; and surabgene lomparvovec (ABBV-RGX-314) for the treatment of wet AMD and diabetic retinopathy, in collaboration with AbbVie. Thousands of patients have been treated with REGENXBIO’s AAV platform, including those receiving Novartis’ ZOLGENSMA®. REGENXBIO’s investigational gene therapies have the potential to change the way healthcare is delivered for millions of people. For more information, please visit www.REGENXBIO.com.
FORWARD-LOOKING STATEMENTS
This press release includes “forward-looking statements,” within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. These statements express a belief, expectation or intention and are generally accompanied by words that convey projected future events or outcomes such as “believe,” “may,” “will,” “estimate,” “continue,” “anticipate,” “assume,” “design,” “intend,” “expect,” “could,” “plan,” “potential,” “predict,” “seek,” “should,” “would” or by variations of such words or by similar expressions. The forward-looking statements include statements relating to, among other things, REGENXBIO’s future operations, clinical trials, costs and cash flow. REGENXBIO has based these forward-looking statements on its current expectations and assumptions and analyses made by REGENXBIO in light of its experience and its perception of historical trends, current conditions and expected future developments, as well as other factors REGENXBIO believes are appropriate under the circumstances. However, whether actual results and developments will conform with REGENXBIO’s expectations and predictions is subject to a number of risks and uncertainties, including the timing of enrollment, commencement and completion and the success of clinical trials conducted by REGENXBIO, its licensees and its partners, the timing of commencement and completion and the success of preclinical studies conducted by REGENXBIO and its development partners, the timing or likelihood of payments from AbbVie or Nippon Shinyaku, the monetization of any priority review voucher, the timely development and launch of new products, the ability to obtain and maintain regulatory approval of product candidates, the ability to obtain and maintain intellectual property protection for product candidates and technology, trends and challenges in the business and markets in which REGENXBIO operates, the size and growth of potential markets for product candidates and the ability to serve those markets, the rate and degree of acceptance of product candidates, and other factors, many of which are beyond the control of REGENXBIO. Refer to the “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of REGENXBIO’s Annual Report on Form 10-K for the year ended December 31, 2024, and comparable “risk factors” sections of REGENXBIO’s Quarterly Reports on Form 10-Q and other filings, which have been filed with the SEC and are available on the SEC’s website at WWW.SEC.GOV. All of the forward-looking statements made in this press release are expressly qualified by the cautionary statements contained or referred to herein. The actual results or developments anticipated may not be realized or, even if substantially realized, they may not have the expected consequences to or effects on REGENXBIO or its businesses or operations. Such statements are not guarantees of future performance and actual results or developments may differ materially from those projected in the forward-looking statements. Readers are cautioned not to rely too heavily on the forward-looking statements contained in this press release. These forward-looking statements speak only as of the date of this press release. Except as required by law, REGENXBIO does not undertake any obligation, and specifically declines any obligation, to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
Zolgensma® is a registered trademark of Novartis Gene Therapies. All other trademarks referenced herein are registered trademarks of REGENXBIO.
CONTACTS:
Dana Cormack
Corporate Communications
[email protected]
George E. MacDougall
Investor Relations
[email protected]
SOURCE REGENXBIO Inc.

The U.S. Food and Drug Administration today announced it is sharing information about the agency’s flexible approach to overseeing chemistry, manufacturing and control (CMC) requirements for cell and gene therapies (CGT). The agency’s more flexible approach has been, and is expected to continue to be, helpful in expediting product development and will help guide the FDA’s evaluation of development strategies in preparation for a Biologics License Application (BLA) submission.
“Regulatory flexibility must be tailored for cell and gene therapies,” said FDA Commissioner Marty Makary, M.D., M.P.H. “These are common-sense reforms that will address the unique characteristics of cell and gene therapies and foster more innovation.”
Over the last decade, the FDA’s Center for Biologics Evaluation and Research (CBER) has approved close to 50 CGTs. The transformative potential of these therapies has captured the imagination of the patient community and ignited product development.
“There has been tremendous enthusiasm amongst product developers resulting in an explosive growth of cell and gene therapy submissions, many of which target serious or life-threatening conditions with an unmet medical need,” Vinay Prasad, M.D., M.P.H., Chief Medical and Scientific Officer and Director of the FDA’s Center for Biologics Evaluation and Research. “CBER is eager for stakeholders to know that our effectiveness at exercising greater regulatory flexibility around chemistry, manufacturing and control requirements furthers innovative product development.”
CBER has historically had similar CMC expectations across products, including small-batch products such as CGTs. CGTs are inherently complex biologic products, often individualized for patients, and may need sophisticated manufacturing under particular time constraints. CBER has leveraged its growing experience with CGT products to identify and implement regulatory flexibilities allowed under FDA’s regulations that accommodate the unique characteristics of these innovative therapies, while maintaining rigorous quality standards through appropriate control measures. While there is a long history of making concerted efforts to help sponsors meet standards to assure product safety, purity and potency, the application of flexibilities has not always been fully clear to stakeholders.
“CBER is proactively communicating about regulatory flexibilities that were previously applied case-by-case to select CGT therapies. By communicating these approaches broadly, we aim to expedite product development across the CGT field,” said Vijay Kumar M.D., Acting Director, Office of Therapeutic Products in the FDA’s Center for Biologics Evaluation and Research. “It is vital that every sponsor, no matter the CBER reviewer team they engage with, understand what types of regulatory flexibility may be scientifically acceptable.”
Given the rapid scientific developments witnessed during the decade, it is a high priority for both the agency and the administration to remove barriers and perceived misconceptions that stand in the way of expedited product development. These flexibilities will enable progress while not compromising or undermining the FDA’s ability to assure safety, purity and potency of a product, or weaken the FDA’s dependency on understanding the benefits and risks of both the specific therapy and the disease context.
In June, the FDA hosted a Cell and Gene Therapy Roundtable, bringing together leading experts to discuss advancing the field of cell and gene therapies for patients and innovators.
###
Boilerplate
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices. The agency also is responsible for the safety and security of our nation’s food supply, cosmetics, dietary supplements, radiation-emitting electronic products, and for regulating tobacco products.

Good afternoon everybody and welcome along to this MARCA in English live blog, this time for the Supercopa de Espana final of Barcelona vs Real Madrid.
Spanish football’s greatest rivalry takes centre stage once again this Sunday evening as…

Prior to Brie’s three-year-old son starting kindergarten, he had never been in the care of anyone other than his parents.
“Any new environment, or environment without us, obviously impacted him greatly,” says the 44-year-old from Geelong/Djilang,…