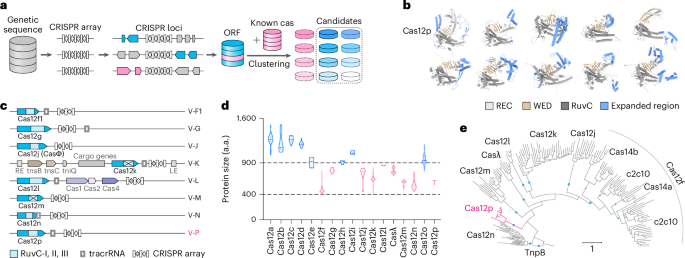
Wiedenheft, B., Sternberg, S. H. & Doudna, J. A. RNA-guided genetic silencing systems in bacteria and archaea. Nature 482, 331–338 (2012).
Google Scholar
Barrangou, R. et al. CRISPR provides acquired resistance against viruses in prokaryotes. Science 315, 1709–1712 (2007).
Google Scholar
Garneau, J. E. et al. The CRISPR–Cas bacterial immune system cleaves bacteriophage and plasmid DNA. Nature 468, 67–71 (2010).
Google Scholar
Marraffini, L. A. CRISPR–Cas immunity in prokaryotes. Nature 526, 55–61 (2015).
Google Scholar
Zhang, S. et al. Pro-CRISPR PcrIIC1-associated Cas9 system for enhanced bacterial immunity. Nature 630, 484–492 (2024).
Google Scholar
Rauch, B. J. et al. Inhibition of CRISPR–Cas9 with bacteriophage proteins. Cell 168, 150–158.e10 (2017).
Google Scholar
Camara-Wilpert, S. et al. Bacteriophages suppress CRISPR–Cas immunity using RNA-based anti-CRISPRs. Nature 623, 601–607 (2023).
Google Scholar
Marino, N. D. et al. Discovery of widespread type I and type V CRISPR–Cas inhibitors. Science 362, 240–242 (2018).
Google Scholar
Bondy-Denomy, J. et al. Multiple mechanisms for CRISPR–Cas inhibition by anti-CRISPR proteins. Nature 526, 136–139 (2015).
Google Scholar
Jia, N. & Patel, D. J. Structure-based functional mechanisms and biotechnology applications of anti-CRISPR proteins. Nat. Rev. Mol. Cell Biol. 22, 563–579 (2021).
Google Scholar
Shu, X. et al. CRISPR-repressed toxin–antitoxin provides herd immunity against anti-CRISPR elements. Nat. Chem. Biol. 21, 337–347 (2025).
Google Scholar
Makarova, K. S. et al. Evolutionary classification of CRISPR–Cas systems: a burst of class 2 and derived variants. Nat. Rev. Microbiol. 18, 67–83 (2020).
Google Scholar
Shmakov, S. et al. Diversity and evolution of class 2 CRISPR–Cas systems. Nat. Rev. Microbiol. 15, 169–182 (2017).
Google Scholar
Wang, J. Y., Pausch, P. & Doudna, J. A. Structural biology of CRISPR–Cas immunity and genome editing enzymes. Nat. Rev. Microbiol. 20, 641–656 (2022).
Google Scholar
Strecker, J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 365, 48–53 (2019).
Google Scholar
Wu, Z. et al. Structure and engineering of miniature Acidibacillus sulfuroxidans Cas12f1. Nat. Catal. 6, 695–709 (2023).
Google Scholar
Takeda, S. N. et al. Structure of the miniature type V-F CRISPR–Cas effector enzyme. Mol. Cell 81, 558–570.e3 (2021).
Google Scholar
Wu, W. Y. et al. The miniature CRISPR–Cas12m effector binds DNA to block transcription. Mol. Cell 82, 4487–4502.e7 (2022).
Google Scholar
Yan, W. X. et al. Functionally diverse type V CRISPR–Cas systems. Science 363, 88–91 (2019).
Google Scholar
Dmytrenko, O. et al. Cas12a2 elicits abortive infection through RNA-triggered destruction of dsDNA. Nature 613, 588–594 (2023).
Google Scholar
Guo, J. et al. VirSorter2: a multi-classifier, expert-guided approach to detect diverse DNA and RNA viruses. Microbiome 9, 37 (2021).
Google Scholar
Camargo, A. P. et al. Identification of mobile genetic elements with geNomad. Nat. Biotechnol. 42, 1303–1312 (2024).
Google Scholar
Zhang, R. et al. SpacePHARER: sensitive identification of phages from CRISPR spacers in prokaryotic hosts. Bioinformatics 37, 3364–3366 (2021).
Google Scholar
Wu, Z. et al. Programmed genome editing by a miniature CRISPR–Cas12f nuclease. Nat. Chem. Biol. 17, 1132–1138 (2021).
Google Scholar
Holmgren, A. Thioredoxin. Annu. Rev. Biochem. 54, 237–271 (1985).
Google Scholar
Martin, J. L. Thioredoxin—a fold for all reasons. Structure 3, 245–250 (1995).
Google Scholar
Zeller, T. & Klug, G. Thioredoxins in bacteria: functions in oxidative stress response and regulation of thioredoxin genes. Naturwissenschaften 93, 259–266 (2006).
Google Scholar
Chartron, J., Shiau, C., Stout, C. D. & Carroll, K. S. 3′-Phosphoadenosine-5′-phosphosulfate reductase in complex with thioredoxin: a structural snapshot in the catalytic cycle. Biochemistry 46, 3942–3951 (2007).
Google Scholar
Hwang, J. et al. The structural basis for the negative regulation of thioredoxin by thioredoxin-interacting protein. Nat. Commun. 5, 2958 (2014).
Google Scholar
Zhang, Z. et al. Structural basis for thioredoxin-mediated suppression of NLRP1 inflammasome. Nature 622, 188–194 (2023).
Google Scholar
Ball, D. P. et al. Oxidized thioredoxin-1 restrains the NLRP1 inflammasome. Sci. Immunol. 7, eabm7200 (2022).
Google Scholar
Gao, Y. et al. Structures and operating principles of the replisome. Science 363, eaav7003 (2019).
Google Scholar
Tabor, S., Huber, H. E. & Richardson, C. C. Escherichia coli thioredoxin confers processivity on the DNA polymerase activity of the gene 5 protein of bacteriophage T7. J. Biol. Chem. 262, 16212–16223 (1987).
Google Scholar
Park, J.-U. et al. Structures of the holo CRISPR RNA-guided transposon integration complex. Nature 613, 775–782 (2023).
Google Scholar
Nakagawa, R. et al. Cryo-EM structure of the transposon-associated TnpB enzyme. Nature 616, 390–397 (2023).
Google Scholar
Yamano, T. et al. Crystal structure of Cpf1 in complex with guide RNA and target DNA. Cell 165, 949–962 (2016).
Google Scholar
Yang, H., Gao, P., Rajashankar, K. R. & Patel, D. J. PAM-dependent target DNA recognition and cleavage by C2c1 CRISPR–cas endonuclease. Cell 167, 1814–1828.e12 (2016).
Google Scholar
Kurihara, N. et al. Structure of the type V-C CRISPR–Cas effector enzyme. Mol. Cell 82, 1865–1877.e4 (2022).
Google Scholar
Tsuchida, C. A. et al. Chimeric CRISPR–CasX enzymes and guide RNAs for improved genome editing activity. Mol. Cell 82, 1199–1209.e6 (2022).
Google Scholar
Hino, T. et al. An AsCas12f-based compact genome-editing tool derived by deep mutational scanning and structural analysis. Cell 186, 4920–4935.e23 (2023).
Google Scholar
Li, Z., Zhang, H., Xiao, R., Han, R. & Chang, L. Cryo-EM structure of the RNA-guided ribonuclease Cas12g. Nat. Chem. Biol. 17, 387–393 (2021).
Google Scholar
Zhang, B. et al. Mechanistic insights into the R-loop formation and cleavage in CRISPR–Cas12i1. Nat. Commun. 12, 3476 (2021).
Google Scholar
Pausch, P. et al. DNA interference states of the hypercompact CRISPR–CasΦ effector. Nat. Struct. Mol. Biol. 28, 652–661 (2021).
Google Scholar
Sun, A. et al. The compact Casπ (Cas12l) ‘bracelet’ provides a unique structural platform for DNA manipulation. Cell Res. 33, 229–244 (2023).
Google Scholar
Omura, S. N. et al. Mechanistic and evolutionary insights into a type V-M CRISPR–Cas effector enzyme. Nat. Struct. Mol. Biol. 30, 1172–1182 (2023).
Google Scholar
Al-Shayeb, B. et al. Diverse virus-encoded CRISPR–Cas systems include streamlined genome editors. Cell 185, 4574–4586.e16 (2022).
Google Scholar
Duan, Z. et al. Structure and genome editing activity of the novel CRISPR–Cas12o1 effector. Cell Res. 35, 145–148 (2025).
Google Scholar
Sasnauskas, G. et al. TnpB structure reveals minimal functional core of Cas12 nuclease family. Nature 616, 384–389 (2023).
Google Scholar
Chamberlin, M. Isolation and characterization of prototrophic mutants of Escherichia coli unable to support the intracellular growth of T7. J. Virol. 14, 509–516 (1974).
Google Scholar
Hamdan, S. M. et al. A unique loop in T7 DNA polymerase mediates the binding of helicase–primase, DNA binding protein, and processivity factor. Proc. Natl Acad. Sci. USA 102, 5096–5101 (2005).
Google Scholar
Evans, R. et al. Protein complex prediction with AlphaFold-Multimer. Preprint at bioRxiv https://doi.org/10.1101/2021.10.04.463034 (2021).
Omidi, A., Møller, M. H., Malhis, N., Bui, J. M. & Gsponer, J. AlphaFold-Multimer accurately captures interactions and dynamics of intrinsically disordered protein regions. Proc. Natl Acad. Sci. USA 121, e2406407121 (2024).
Google Scholar
Wilson, C. J., Choy, W.-Y. & Karttunen, M. AlphaFold2: a role for disordered protein/region prediction?. Int. J. Mol. Sci. 23, 4591 (2022).
Google Scholar
Holehouse, A. S. & Kragelund, B. B. The molecular basis for cellular function of intrinsically disordered protein regions. Nat. Rev. Mol. Cell Biol. 25, 187–211 (2024).
Google Scholar
Bryant, P., Pozzati, G. & Elofsson, A. Improved prediction of protein-protein interactions using AlphaFold2. Nat. Commun. 13, 1265 (2022).
Google Scholar
Tang, N. & Ji, Q. Miniature CRISPR–Cas12 systems: mechanisms, engineering, and genome editing applications. ACS Chem. Biol. 19, 1399–1408 (2024).
Google Scholar
Karvelis, T. et al. Transposon-associated TnpB is a programmable RNA-guided DNA endonuclease. Nature 599, 692–696 (2021).
Google Scholar
Wu, W. Y., Adiego-Pérez, B. & Van Der Oost, J. Biology and applications of CRISPR–Cas12 and transposon-associated homologs. Nat. Biotechnol. 42, 1807–1821 (2024).
Google Scholar
Chen, W. et al. Cas12n nucleases, early evolutionary intermediates of type V CRISPR, comprise a distinct family of miniature genome editors. Mol. Cell 83, 2768–2780.e6 (2023).
Google Scholar
Xu, X. et al. Engineered miniature CRISPR–Cas system for mammalian genome regulation and editing. Mol. Cell 81, 4333–4345.e4 (2021).
Google Scholar
Kim, D. Y. et al. Efficient CRISPR editing with a hypercompact Cas12f1 and engineered guide RNAs delivered by adeno-associated virus. Nat. Biotechnol. 40, 94–102 (2022).
Google Scholar
Bland, C. et al. CRISPR Recognition Tool (CRT): a tool for automatic detection of clustered regularly interspaced palindromic repeats. BMC Bioinformatics 8, 209 (2007).
Google Scholar
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinformatics 11, 119 (2010).
Google Scholar
Pausch, P. et al. CRISPR–CasΦ from huge phages is a hypercompact genome editor. Science 369, 333–337 (2020).
Google Scholar
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Google Scholar
Eddy, S. R. Accelerated profile HMM searches. PLoS Comput. Biol. 7, e1002195 (2011).
Google Scholar
Steinegger, M. & Söding, J. MMseqs2 enables sensitive protein sequence searching for the analysis of massive data sets. Nat. Biotechnol. 35, 1026–1028 (2017).
Google Scholar
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Google Scholar
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Google Scholar
Edgar, R. C. Muscle5: high-accuracy alignment ensembles enable unbiased assessments of sequence homology and phylogeny. Nat. Commun. 13, 6968 (2022).
Google Scholar
Minh, B. Q. et al. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 37, 1530–1534 (2020).
Google Scholar
Letunic, I. & Bork, P. Interactive Tree of Life (iTOL) v6: recent updates to the phylogenetic tree display and annotation tool. Nucleic Acids Res. 52, W78–W82 (2024).
Google Scholar
Yu, D., Chojnowski, G., Rosenthal, M. & Kosinski, J. AlphaPulldown—a Python package for protein–protein interaction screens using AlphaFold-Multimer. Bioinformatics 39, btac749 (2023).
Google Scholar
Wang, Y. et al. CRISPR–Cas9 and CRISPR-assisted cytidine deaminase enable precise and efficient genome editing in Klebsiella pneumoniae. Appl. Environ. Microbiol. 84, e01834–18 (2018).
Google Scholar
Wang, Y. et al. A highly efficient CRISPR–Cas9-based genome engineering platform in Acinetobacter baumannii to understand the H2O2-sensing mechanism of OxyR. Cell Chem. Biol. 26, 1732–1742.e5 (2019).
Google Scholar
Quan, J. & Tian, J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 4, e6441 (2009).
Google Scholar
Zhu, J. et al. Design of bacteriophage T4-based artificial viral vectors for human genome remodeling. Nat. Commun. 14, 2928 (2023).
He, Y. & Chen, J. CRISPR–Cas9-mediated genome editing of T4 bacteriophage for high-throughput antimicrobial susceptibility testing. Anal. Chem. 96, 18301–18310 (2024).
Google Scholar
Kim, D., Paggi, J. M., Park, C., Bennett, C. & Salzberg, S. L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 37, 907–915 (2019).
Google Scholar
Danecek, P. et al. Twelve years of SAMtools and BCFtools. GigaScience 10, giab008 (2021).
Mastronarde, D. N. Automated electron microscope tomography using robust prediction of specimen movements. J. Struct. Biol. 152, 36–51 (2005).
Google Scholar
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Google Scholar
Zivanov, J. et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife 7, e42166 (2018).
Google Scholar
Punjani, A., Zhang, H. & Fleet, D. J. Non-uniform refinement: adaptive regularization improves single-particle cryo-EM reconstruction. Nat. Methods 17, 1214–1221 (2020).
Google Scholar
Punjani, A., Rubinstein, J. L., Fleet, D. J. & Brubaker, M. A. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods 14, 290–296 (2017).
Google Scholar
Sanchez-Garcia, R. et al. DeepEMhancer: a deep learning solution for cryo-EM volume post-processing. Commun. Biol. 4, 874 (2021).
Google Scholar
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D 60, 2126–2132 (2004).
Google Scholar
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Google Scholar
Meng, E. C. et al. UCSF ChimeraX: tools for structure building and analysis. Protein Sci. 32, e4792 (2023).
Google Scholar