- Ethiopia strikes draft restructuring deal on $1 billion bond with bondholder group Reuters
- Ethiopia nears $1b bond restructuring The Express Tribune
- Ethiopia reaches agreement in principle with Ad Hoc Committee of bondholders on principal financial terms of restructuring of 2024 Notes- Ministry of Finance – Addis Media Network -English አዲስ ሚዲያ ኔትወርክ
- BREAKING NEWS: Ethiopia Reaches Deal in Principle on $1bln Eurobond Birr Metrics
- News: #Ethiopia reaches preliminary deal with bondholders on restructuring of 2024 #Eurobond Ethiopia has reached an agreement in principle with a group of international bondholders on the main financial terms for restructuring its US$1 billion Eurobond th facebook.com
Author: admin
-
Ethiopia strikes draft restructuring deal on $1 billion bond with bondholder group – Reuters
-

‘Stranger Things’ creators respond to burning question about finale
Did Elven die in the finale of Stranger Things? Unsure fans took to social…
Continue Reading
-

Bella Hadid’s Off-Duty Model Wardrobe Is Even Better in Aspen
On another day, Hadid was spotted experimenting in a more divisive denim trend that’s been quietly taking over—the Gen-X boot tuck. Hadid wore straight-leg blue denim jeans tucked into some patent leather knee-high Pucci boots—shoes…
Continue Reading
-

Meanwood Valley Trail in Leeds to be made more accessible
 Elizabeth Baines / BBC
Elizabeth Baines / BBCSally Morgan says paths along the Meanwood Valley Trail are crumbling. Funding has been secured for a series of upgrades to the Meanwood Valley Trail – a seven-mile long “green artery” which runs through Leeds from built-up…
Continue Reading
-
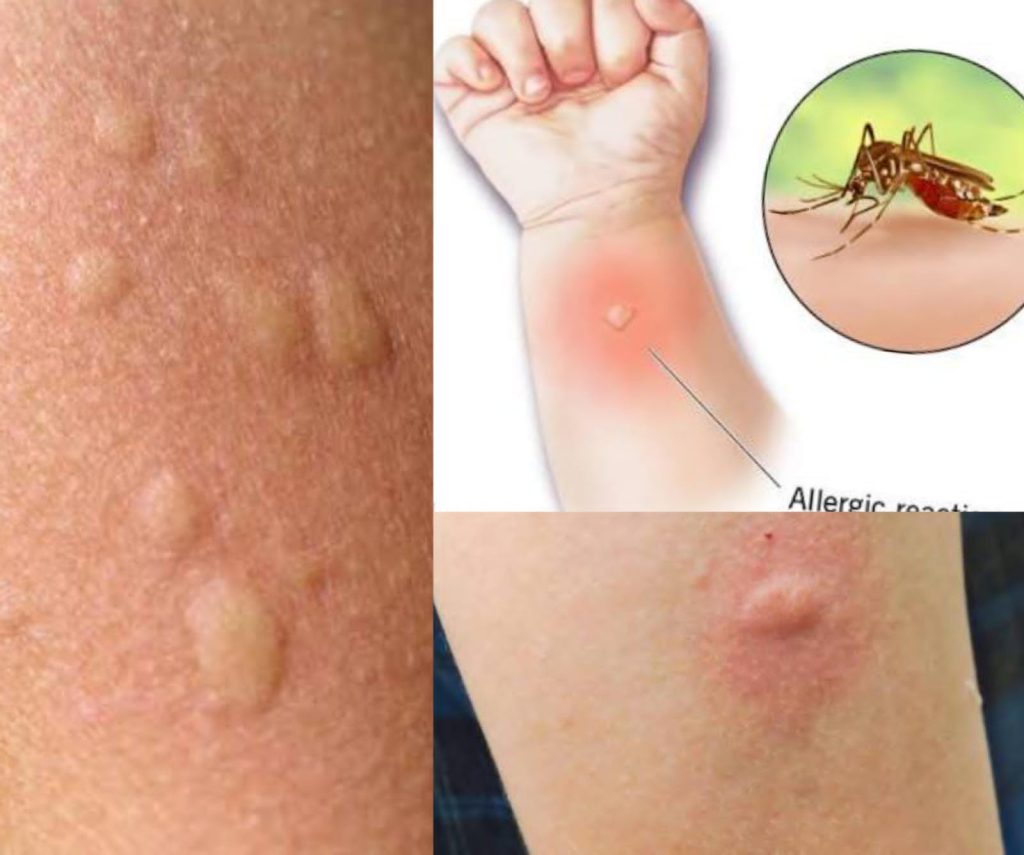
Mosquito Menace Worsens in Latifabad, Residents Fear Disease Outbreak – https://www.dailyindependent.com.pk
Mosquito Menace Worsens in Latifabad, Residents Fear Disease Outbreak – https://www.dailyindependent.com.pk
Continue Reading
-

The rise and fall of the in-flight telephone
Long before WiFi icons appeared on seatback screens,…
Continue Reading
-
SLLEA AC/DC Adapter for Le Pan Mini TC802A TC802 Android Touch Screen Tablet Power Supply Cord Cable PS Charger Mains PSU
Product Details
- World Wide Input Voltage 100-240VAC 50/60Hz
- SLLEA–Determined to become the most professional store of wire and chargers
- Industry Quality : Over Voltage Protection, Over Heat Protection
- Note: Please make sure the model of your device whether it is the same as the listing shows before purchasing
- Warranty: 30 Days Money Back Guarantee / 60 Days Free Exchange With Paid Return Label / 360 Days Anytime Worry-Free Warranty! /
Please don’t hesitate to contact us if any questions or concerns – we are here to help!
Product Description
SLLEA AC / DC Adapter For Le Pan mini TC802A TC802 Android Touch Screen Tablet Power Supply Cord Cable PS Charger Mains PSU
Continue Reading
-
Panama reports 50% decrease in dengue in 2025
The Panama Ministry of Health report 15,657 dengue fever cases mid-December 2025, while for the same week in 2024, approximately 32,000 cases were registered, reflecting a significant reduction, more than 50 percent.
Much can be said concerning…
Continue Reading
-
Real Madrid's Alonso unsure of Mbappe absence length – France 24
- Real Madrid’s Alonso unsure of Mbappe absence length France 24
- Xabi opens up on Mbappe’s return The Express Tribune
- Mbappé medical report realmadrid.com
- Alonso talks Carvajal, Mbappe, Vinicius, Huijsen, Endrick ahead of Real Madrid vs Betis
Continue Reading
-

The OncFive: Top Oncology Articles for the Week of 12/28
Welcome to OncLive®’s OncFive!
Every week, we bring you a quick roundup of the 5 top stories from the world of oncology—ranging from pivotal regulatory decisions to key pipeline updates to expert insights on breakthroughs that are moving the needle in cancer care. This resource is designed to keep you informed on the latest updates in the space, in just a matter of minutes.
Here’s what you may have missed this week:
The FDA approved narsoplimab-wuug (Yartemlea) for the treatment of adults and pediatric patients aged 2 years or older with hematopoietic stem cell transplant–associated thrombotic microangiopathy (TA-TMA). The decision was supported by single-arm TA-TMA Study and expanded access program data showing a TMA complete response (CR) rate of 61% in evaluable patients, with consistent responses across adult and pediatric cohorts. Treatment led to improvements in platelet count, lactate dehydrogenase levels, organ function, and transfusion independence, with a 100-day survival rate of approximately 73% from TMA diagnosis. This marks the first FDA-approved therapy for TA-TMA, representing a practice-changing advance in post-transplant supportive care.
A new drug application has been submitted to the FDA seeking approval of bezuclastinib (CGT0486) for use in patients with nonadvanced systemic mastocytosis, based on positive results from the phase 2 SUMMIT trial (NCT05186753). Bezuclastinib significantly improved total symptom score at week 24 compared with placebo and led to higher rates of clinically meaningful symptom reduction. The agent also showcased marked biologic activity, with over 95% of evaluable patients achieving at least a 50% reduction in serum tryptase levels. These data support bezuclastinib as a potential disease-modifying therapy in a population with limited approved options.
China’s National Medical Products Administration approved ipilimumab N01 (IBI310; Tabosun) in combination with sintilimab (Tyvyt) as neoadjuvant therapy for patients with stage IIB to III resectable microsatellite instability–high/mismatch repair–deficient colon cancer. The decision was supported by data from the phase 3 NeoShot study (NCT05890742) demonstrating an 82% pathological CR (pCR) rate with the combination versus surgery alone, without increased surgical risk. Earlier phase 1b results showed significantly higher pCR rates with ipilimumab N01 plus sintilimab vs sintilimab monotherapy. This represents the world’s first approval of a CTLA-4 antibody in the neoadjuvant colon cancer setting.
Japan’s Ministry of Health, Labour and Welfare approved tafasitamab-cxix (Minjuvi) plus rituximab (Rituxan) and lenalidomide (Revlimid) for the treatment of adults with relapsed or refractory follicular lymphoma. The decision was based on findings from the phase 3 inMIND trial (NCT04680052), which showed a significant progression-free survival (PFS) benefit over placebo plus rituximab and lenalidomide, at a median PFS of 22.4 months vs 13.9 months (HR, 0.43). Independent review confirmed durable PFS benefit, with median PFS not reached in the tafasitamab arm. This approval establishes a chemotherapy-free, dual CD19/CD20–targeted option in Japan for relapsed/refractory disease.
In December 2025, the FDA issued multiple oncology approvals, including full approval of pirtobrutinib (Jaypirca) for relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma based on findings from BRUIN CLL-321 (NCT04666038) and clearance of lisocabtagene maraleucel (Breyanzi) for relapsed/refractory marginal zone lymphoma based on data from TRANSCEND-FL (NCT04245839). Additional approvals included niraparib plus abiraterone (Akeega) for BRCA2-mutated metastatic castration-sensitive prostate cancer based on findings from AMPLITUDE (NCT04497844) and fam-trastuzumab deruxtecan-nxki (Enhertu) plus pertuzumab (Perjeta) for frontline HER2-positive metastatic breast cancer based on data from DESTINY-Breast09 (NCT04784715). The agency also expanded access to subcutaneous formulations of amivantamab and hyaluronidase-lpuj (Rybrevant Faspro) and mosunetuzumab (Lunsumio VELO), improving treatment convenience without compromising efficacy. Collectively, these decisions reflect continued momentum toward precision oncology, novel drug delivery platforms, and earlier use of targeted and cellular therapies.
Continue Reading