- New Zealand mosque shooter’s former lawyers say he showed anxiety but did not appear depressed Reuters
- New Zealand’s Christchurch mosque killer appeals conviction Dawn
- Christchurch shooter seeks to overturn guilty plea BBC
- New Zealand mosque…
Author: admin
-
New Zealand mosque shooter's former lawyers say he showed anxiety but did not appear depressed – Reuters
-
World Pulses Day being observed today – RADIO PAKISTAN
- World Pulses Day being observed today RADIO PAKISTAN
- A Beans’ Story on International Pulses Day Slow Food
- The Humble Daal, the Mighty Impact: World Pulses Day Daily Pioneer
- LoP Naveen Patnaik Urges Pulse Promotion For Health And Planet Ommcom…
Continue Reading
-

New spatial omics platform advances biomedical research in Spain
Spatial omics is a new generation of technologies that allow scientists to study cells in their original location within tissues (without isolating or moving them from their environment). Just as a city cannot be understood by…
Continue Reading
-
Iran offers concessions on nuclear program – Arab News
- Iran offers concessions on nuclear program Arab News
- Iran says it could dilute enriched uranium if all sanctions are lifted Dawn
- Iran Vice President Unveils Details of Negotiations with US Caspian Post
- Iran Update, February 9, 2026 Institute for…
Continue Reading
-
President Zardari, Faisal Vawda discuss political situation – RADIO PAKISTAN
- President Zardari, Faisal Vawda discuss political situation RADIO PAKISTAN
- Senator Faisal Vawda meets President Associated Press of Pakistan
- Senator Faisal Vawda meets President Zardari The Nation (Pakistan )
- Faisal Vawda meets President,…
Continue Reading
-

India-Pakistan clash back on after boycott ends
The high-stakes match between India and Pakistan at the T20 World Cup is back on after the Pakistani government on Monday directed its national cricket team to play India.
The announcement ends a week-long boycott that had threatened one of…
Continue Reading
-

Physicists discover what controls the speed of quantum time
“The concept of time has troubled philosophers and physicists for thousands of years, and the advent of quantum mechanics has not simplified the problem,” says Professor Hugo Dil, a physicist at EPFL. “The central problem is the general role of…
Continue Reading
-

Super People Shuts Down After Player Counts Dwindle—Again
Super People, a once-popular battle royale game that takes the typical first- and third-person shooter gameplay and throws superpowers into the mix, is no stranger to hard times, with the battle royale having already suffered a shut-down once…Continue Reading
-
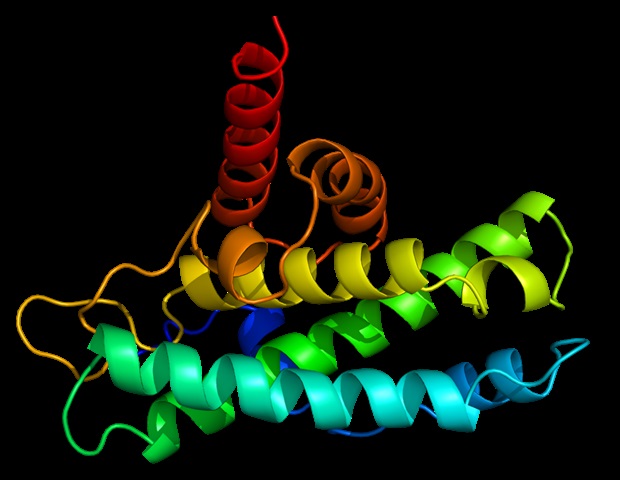
Newly identified protein interaction fine-tunes cellular stress responses
Cornell researchers have discovered a new way cells regulate how they respond to stress, identifying an interaction between two proteins that helps keep a critical cellular recycling system in balance.
The findings show that a…
Continue Reading
-

I turned doodles into video clips in minutes with Runway’s new AI video tool – here’s how
AI transformed doodles of birds into scary winged creatures.
Webb Wright/ZDNET/via Runway AI
Follow ZDNET: Add us as a preferred source on Google.
ZDNET’s key takeaways
- A new feature from Runway lets you generate video from doodles.
- It’s…
Continue Reading