Fraz, 22, Bradford
Occupation Law graduate, training to become a commercial solicitor
Voting record Was only eligible to vote in the last election and had to go away at the last minute. Describes himself as “pretty central”
Amuse bouche When…

Occupation Law graduate, training to become a commercial solicitor
Voting record Was only eligible to vote in the last election and had to go away at the last minute. Describes himself as “pretty central”
Amuse bouche When…

An ancient tomb discovered in Turkey may have been made for a member of the family of the legendary King Midas, who lived in the eighth century B.C. and is renowned for his mythical “golden touch.”
The possibly royal tomb, from the ancient…

Speediance unveiled its new Gym Nano and Speediance Strap products at CES 2026. The smart fitness equipment manufacturer, which previously developed its own smart home gym, the Gym Monster 2, designed the Gym Nano and Speediance Strap to…
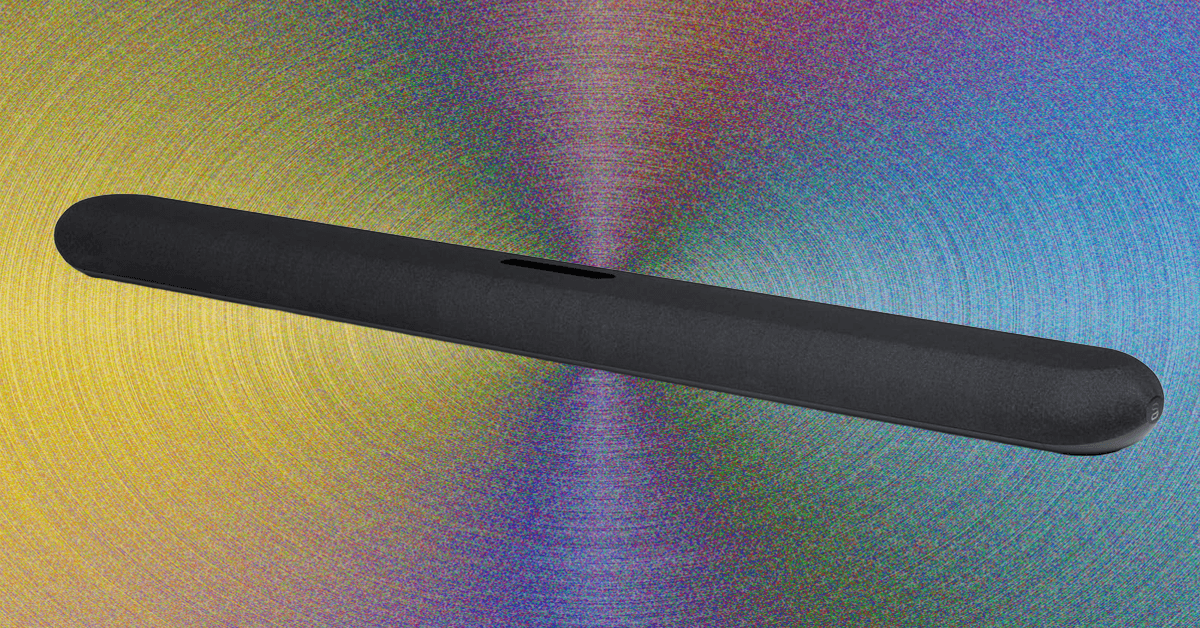
If you’re looking for a top-notch Dolby Atmos soundbar, it’s hard to beat the Sonos Arc Ultra. But Sonos is still recovering from its self-imposed 2024 software fiasco, and many of its once-loyal customers have sought refuge with the…
AstraZeneca reported positive results from a Phase III TULIP-SC clinical trial, showing a statistically significant and clinically meaningful reduction in disease activity with the subcutaneous (SC), self-administration of Saphnelo (anifrolumab) in adults with systemic lupus erythematosus (SLE). The safety profile observed with SC Saphnelo was consistent with the known clinical profile of Saphnelo administered as an intravenous infusion.
In the 52-week study, 56.2% of study participants receiving Saphnelo experienced a significant reduction in lupus disease activity at week 52 compared to 37.1% of participants who received the placebo. Results were measured by the British Isles Lupus Assessment Group-based Composite Lupus Assessment (BICLA). In pre-specified secondary and exploratory endpoints, 29.0% of participants taking Saphnelo achieved remission and 40.1% achieved low disease activity by week 52. SC Saphnelo was well tolerated, with the frequency of overall adverse events balanced between the Saphnelo and placebo groups, SC Saphnelo is approved in the European Union and is under regulatory review in other countries, including the United States and Japan.
Continue to follow the Lupus Foundation of America for updates on lupus drug developments. Learn more about Saphnelo.
Read the announcement
Naturally, perhaps, people building a home theater tend to focus on two things: their smart TV and their sound system. There are other considerations, like decorations and furniture, but some people might gladly watch a movie from a folding…
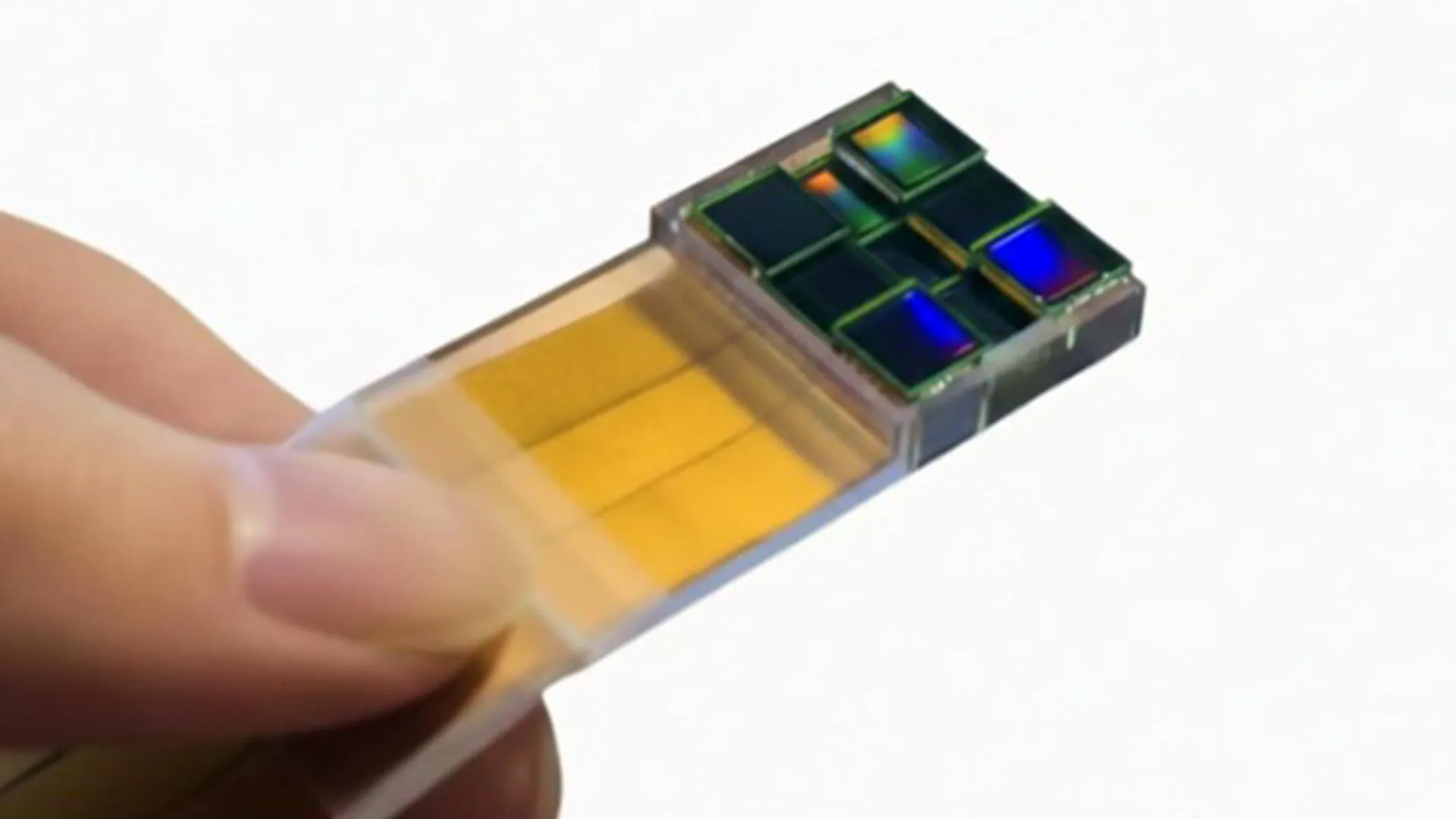
Imaging tools have dramatically reshaped how scientists study the world, from charting faraway galaxies with radio telescope networks to revealing intricate structures inside living cells. Even with decades of progress, one major obstacle has…