SAVE $38.03: As of Jan. 2, the Nothing CMF Watch 3 Pro is down to a new best-ever price at Amazon. Get it for just $60.97 in the light green colorway — a savings of 38%.
…

SAVE $38.03: As of Jan. 2, the Nothing CMF Watch 3 Pro is down to a new best-ever price at Amazon. Get it for just $60.97 in the light green colorway — a savings of 38%.
…

Opticians are starting a challenge to run, walk and cycle 6,214 miles (10,000km) in 2026 – one kilometre for each person estimated to be living with sight loss across Devon and Cornwall.
Optician Tom Mannering said: “This number has real meaning…

#7/6 Maryland (14-1, 2-1) vs. Indiana (11-4, 0-3)
January 4, 2026
6 PM ET
XFINITY…

With the busy holiday season officially behind us, a lot of people are settling back into their regular routines — which, ideally, includes carving out some time to relax with a good book. If you read on a Kindle or use the Kindle app,…

 Carolyn Harris
Carolyn HarrisAn MP who lost nearly 10 stone on Mounjaro has said people who take unregulated weight-loss jabs are “playing Russian roulette…
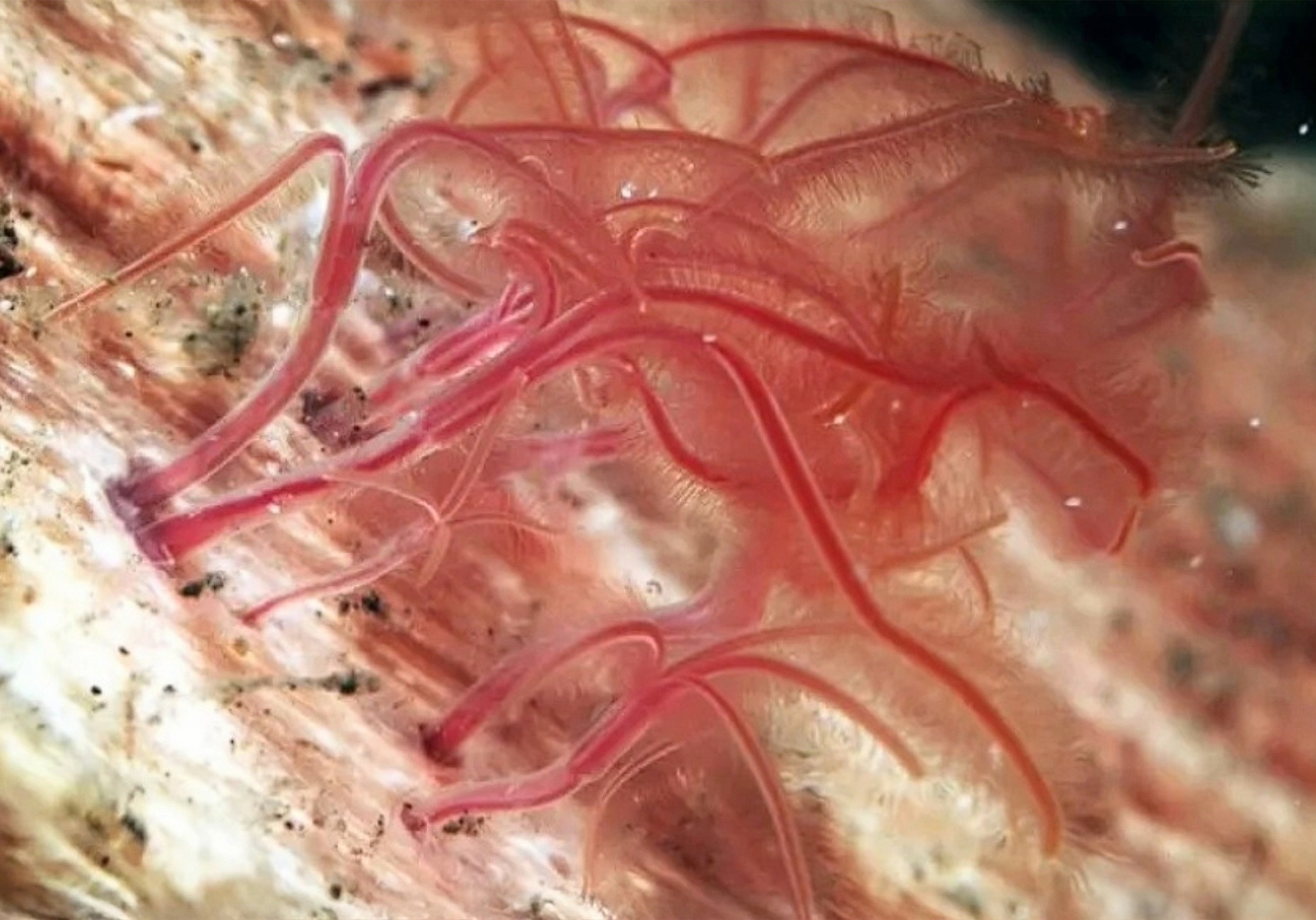
Horror movies are full of monsters you never quite see. Lately, the deep Pacific off British Columbia has its own version of that: a crucial worm that simply isn’t showing up.
The no-show is Osedax – nicknamed the “zombie worm” – a tiny,…
A decade after Saudi Arabia and the United Arab Emirates led a joint military campaign to curb Iran’s influence in Yemen, the two allies now find themselves pitted against each other there.
…


Quantum Biopharma has completed dosing in two toxicology studies requested by the U.S. Food and Drug Administration (FDA) that aim to support the launch of clinical studies of Lucid-MS, an experimental treatment for multiple sclerosis (MS)…
By Matthew Stenger
Posted: 1/2/2026 10:04:00 AM
Last Updated:
In a phase III trial (inMIND) reported in The Lancet, Sehn et al found that the addition of the CD19-targeted Fc-enhanced monoclonal antibody tafasitamab to lenalidomide plus rituximab improved progression-free survival in patients with relapsed or refractory follicular lymphoma.
Study Details
In the global double-blind trial, 548 patients from sites in North America, Europe, and the Asia-Pacific region were randomly assigned between April 2021 and August 2023 to receive up to twelve 28-day cycles of tafasitamab at 12 mg/kg on days 1, 8, 15, and 22 of cycles 1 to 3 and days 1 and 15 of cycles 4 to 12 (n = 273) or placebo (n = 275), with both groups receiving lenalidomide at 20 mg/day on days 1 to 21 of cycles 1 to 12 and rituximab at 375 mg/m² on days 1, 8, 15, and 22 of cycle 1 and day 1 of cycles 2 to 5. In total, 80% of patients were White. The primary endpoint was investigator-assessed progression-free survival in the intention-to-treat population.
Key Findings
Median follow-up was 14.3 months (95% confidence interval [CI] = 11.8–15.0 months) in the tafasitamab group and 14.1 months (95% CI = 11.5–15.0 months) in the control group. Median progression-free survival on investigator assessment was 22.4 months (95% CI = 19.2 months to not evaluable) in the tafasitamab group vs 13.9 months (95% CI = 11.5–16.4 months) in the control group (hazard ratio [HR] = 0.43, 95% CI = 0.32–0.58, P < .0001).
On independent review committee assessment, median progression-free survival was not reached (95% CI = 19.3 months to not evaluable) in the tafasitamab group vs 16.0 months (95% CI = 13.9–21.1 months) in the control group (HR = 0.41, 95% CI = 0.29–0.56, P < .0001).
Death occurred in 15 patients in the tafasitamab group and 23 patients in the control group. Full analysis of overall survival is planned at 5 years of follow-up.
Grade 3 to 4 adverse events occurred in 71% of the tafasitamab group vs 69% of the control group. The most common were neutropenia (40%), pneumonia (8%), COVID-19 (6%), and thrombocytopenia (6%) in the tafasitamab group, and neutropenia (38%), thrombocytopenia (7%), anemia (6%), and pneumonia (5%) in the control group. Serious adverse events occurred in 36% vs 32% of patients, most commonly pneumonia in both groups (8% vs 5%). Adverse events led to the discontinuation of treatment in 11% vs 7% of patients.
The investigators concluded: “The addition of tafasitamab to lenalidomide and rituximab resulted in a statistically significant and clinically meaningful improvement in progression-free survival, with an acceptable safety profile in patients with relapsed or refractory follicular lymphoma. This combination represents a potential new standard-of-care treatment.”
Laurie H. Sehn, MD, of BC Cancer Centre for Lymphoid Cancer and The University of British Columbia, Vancouver, Canada, is the corresponding author for The Lancet article.
Disclosure: The study was funded by Incyte. For full disclosures of the study authors, visit thelancet.com.