- NA passes resolution paying tributes to Armed Forces RADIO PAKISTAN
- ‘US Used Pakistan Then Threw Away Like Toilet Paper’: Khawaja Asif’s Big Admission NDTV
- Khawaja Asif stresses need for national identity as NA passes resolution condemning…
Author: admin
-
NA passes resolution paying tributes to Armed Forces – RADIO PAKISTAN
-

Mosses for Mars: Testing Aquatic Plants as Space-Ready Biofilters
Enabling & Support 11/02/2026
3 views
0 likesLong-duration space missions will require closed-loop life support systems that can regenerate oxygen and purify water while recycling waste. A recent…
Continue Reading
-
Tree of Savior “Revival of Zmei” Major Update
Tree of Savior Official Youtube: https://youtu.be/Q0X2y8f4ddw
Tree of Savior, the MMORPG developed by IMCGames (CEO Hak-kyu Kim) and published on Steam, is now releasing a new episode: “Revival of Zmei”
In this update, the ancient seal…
Continue Reading
-
Akeso’s IL-4Rα/ST2 Bispecific Antibody Cleared for Seven Phase II Studies in China Spanning Respiratory and Autoimmune Indications
HONG KONG, Feb. 11, 2026 /PRNewswire/ — Akeso, Inc. (9926.HK) is pleased to announce that the National Medical Products Administration has approved the initiation of Phase II clinical trials for AK139, a first-in-class IL-4Rα/ST2-targeting bispecific antibody, across seven indications. These indications include chronic obstructive pulmonary disease (COPD), severe bronchial asthma, chronic spontaneous urticaria, allergic rhinitis, chronic rhinosinusitis with nasal polyps, moderate-to-severe atopic dermatitis, and prurigo nodularis. With these new Phase II studies, AK139 has the potential to bring its novel mechanism of action to create breakthrough therapies for multiple respiratory and autoimmune indications
AK139 is a clinical stage bispecific antibody for autoimmune indications that was discovered using Akeso’s proprietary AI-enabled drug discovery platform. It marks a pivotal expansion of the company’s leading expertise in bispecific/multispecific antibody for oncology into other major therapeutic areas. Chronic inflammatory diseases driven by the IL-4Rα/ST2 pathway, including key respiratory and autoimmune disorders, are characterized by complex pathogenesis and a substantial patient burden worldwide. Significant unmet clinical needs persist in many of these indications due to insufficient responses or limited symptom control from current single-target therapies.
As the world’s first IL-4Rα/ST2 bispecific antibody to enter the clinic, AK139 simultaneously targets and blocks both the IL-4/IL-13 pathway (by binding to the IL-4Rα subunit shared by both IL-4 and IL-13 receptor complexes) and the IL-33/ST2-mediated inflammation pathway. Early studies show that AK139 possesses strong bispecific binding affinity, as well as favorable in vitro and in vivo pharmacological activity. In key metrics, including inhibition of inflammatory cytokine release and reduction of tissue inflammatory cell infiltration, AK139 demonstrates significantly greater synergistic efficacy compared to single-target antibodies against either IL-4 or ST2. AK139 also has a good safety profile from earlier studies.
To date, no antibody drug targeting both the IL-4Rαand the IL-33/ST2 pathways has been approved or is in clinical studies. By simultaneously inhibiting these core inflammatory pathways, AK139 has the potential to advance the treatment of related respiratory, autoimmune, and dermatological diseases into a “dual-target era,” offering patients a superior and broad spectrum therapeutic solution. The expansion and advancement of AK139’s global clinical development program will further strengthen Akeso’s momentum in autoimmune diseases. This progress builds upon the foundation established by approved or late-stage novel autoimmune therapies in Akeso’s portfolio, such as ebdarokimab (IL-12/IL-23), gumokimab (IL-17A), and manfidokimab (IL-4R).
Forward-Looking Statement of Akeso, Inc.
This announcement by Akeso, Inc. (9926.HK, “Akeso”) contains “forward-looking statements”. These statements reflect the current beliefs and expectations of Akeso’s management and are subject to significant risks and uncertainties. These statements are not intended to form the basis of any investment decision or any decision to purchase securities of Akeso. There can be no assurance that the drug candidate(s) indicated in this announcement or Akeso’s other pipeline candidates will obtain the required regulatory approvals or achieve commercial success. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in P.R.China, the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; Akeso’s ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the Akeso’s patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
Akeso does not undertake any obligation to publicly revise these forward-looking statements to reflect events or circumstances after the date hereof, except as required by law.
About Akeso
Akeso (HKEX: 9926.HK) is a leading biopharmaceutical company committed to the research, development, manufacturing and commercialization of the world’s first or best-in-class innovative biological medicines. Founded in 2012, the company has established a robust R&D innovation ecosystem centered on its proprietary Tetrabody bispecific antibody platform, ADC (Antibody-Drug Conjugate) technologies, siRNA/mRNA modalities, and cell therapies. Supported by a global-standard GMP manufacturing infrastructure and a highly efficient, integrated commercialization model, the company has evolved into a globally competitive biopharmaceutical focused on innovative solutions. With fully integrated multi-functional platform, Akeso is internally working on a robust pipeline of over 50 innovative assets in the fields of cancer, autoimmune disease, inflammation, metabolic disease and other major diseases. Among them, 26 candidates have entered clinical trials (including 15 bispecific/multispecific antibodies and bispecific ADCs. Additionally, 7 new drugs are commercially available. Through efficient and breakthrough R&D innovation, Akeso always integrates superior global resources, develops the first-in-class and best-in-class new drugs, provides affordable therapeutic antibodies for patients worldwide, and continuously creates more commercial and social values to become a global leading biopharmaceutical enterprise.For more information, please visit https://www.akesobio.com/en/about-us/corporate-profile/ and follow us on Linkedin.
SOURCE Akeso, Inc.

Continue Reading
-
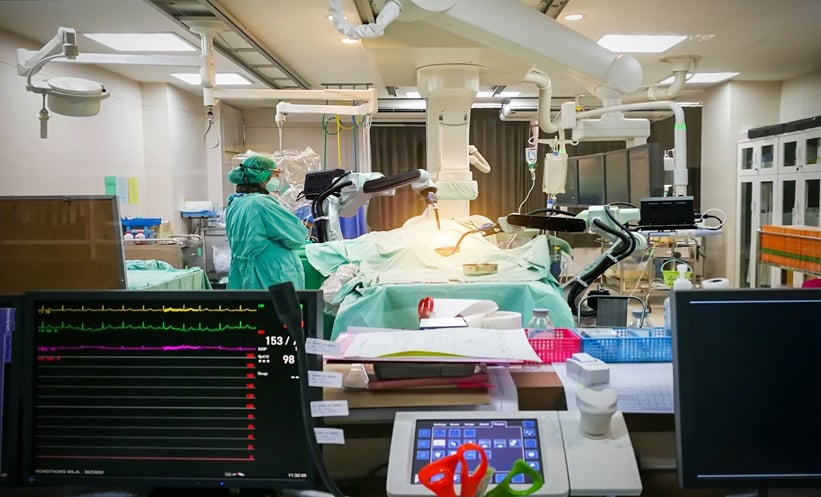
Robotic Secondary Cytoreductive Surgery Outcome
ROBOTIC secondary cytoreductive surgery enabled rapid complete removal of three extrapelvic ovarian cancer recurrences in one case.
Robotic Secondary Cytoreductive Surgery Across Three Sites
Secondary cytoreductive surgery (SCS) is…
Continue Reading
-

Little Amélie review – tender and poignant study of the fragility of early childhood | Film
This tender and sweet animation from film-makers Maïlys Vallade and Liane-Cho Han is an involving, poignant study of early childhood; how fragile it is, and how strong you feel yourself to be to have outlived or surpassed it. It is based on the…
Continue Reading
-

Foote names exciting SA U20 squad for Georgia tour
The SA Rugby U20 squad are scheduled to leave for Georgia on Sunday, 15 February, and will return home on 28 February following the completion of three matches, two of which will be internationals against the…
Continue Reading
-

Jupiter is smaller and more squashed than we thought, says NASA
Jupiter is the biggest planet in our Solar System. It’s so big, in fact, that every other planet of the Solar System could fit inside Jupiter.
Jupiter is a gas giant, which means it’s mostly made of gases like hydrogen and helium surrounding a…
Continue Reading
-

India reduces takedown window to three hours for YouTube, Meta, X and others – BBC
- India reduces takedown window to three hours for YouTube, Meta, X and others BBC
- India tightens grip on social media with new 3-hour takedown rule Dawn
- Big news for Instagram and X users, Modi government tightens rules on Deepfakes, posting AI…
Continue Reading
-

Bad Bunny’s Super Bowl show: Why is Cardi B upsetting traders?
By Euronews with AP
Published on
Cardi B was part of Bad Bunny’s Super Bowl halftime show. But exactly what…
Continue Reading