Construction as a major driver of CO2 emissions and air-pollution health burdens in China.
GA, UNITED STATES, February 11, 2026 /EINPresswire.com/ — Construction-related activities are responsible for roughly half of China’s premature…
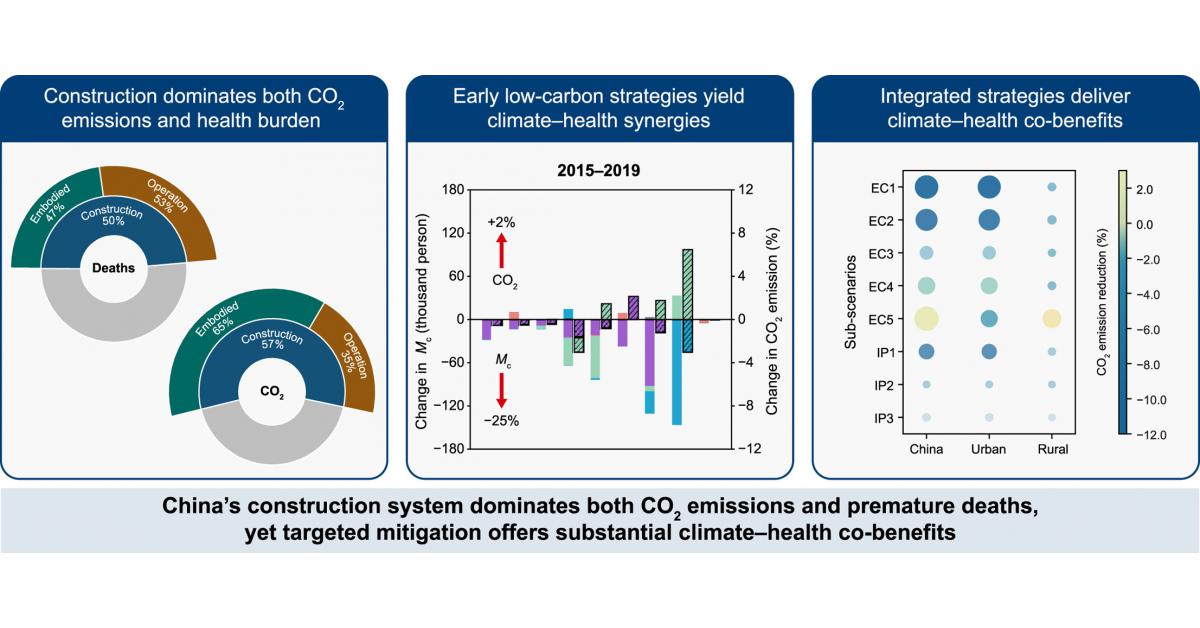
Construction as a major driver of CO2 emissions and air-pollution health burdens in China.
GA, UNITED STATES, February 11, 2026 /EINPresswire.com/ — Construction-related activities are responsible for roughly half of China’s premature…

The Federal Constitutional Court has stopped the Islamabad High Court from proceeding with contempt of court actions against Prime Minister Shehbaz Sharif and federal ministers in the high-profile Aafia Siddiqui case, and issued notices to all…



Master clown and French theatre guru Philippe Gaulier has passed away aged 82, but his influence will live on around the world – particularly in Aotearoa New Zealand.
The performance style inspired by Gaulier can be traced throughout New…

The Lahore Sessions Court on Wednesday extended the interim bail of…

New York – Feb. 11, 2026 – Medidata, a Dassault Systèmes brand and leading provider of clinical trial solutions to the life sciences industry, continues to accelerate clinical trial success for biopharmaceutical and medtech customers through enhanced AI-powered capabilities. Its AI technology has been scaled across the Medidata Platform, benefiting over 500 clinical studies in the last decade, including more than 120 AI-supported studies starting in 2025.
Building on its established AI foundation, Medidata continues to seamlessly weave intelligence into more solutions across its unified platform. The latest advancements include significant enhancements to Medidata Designer with the introduction of Medidata AI Study Build. The new capability accelerates study builds by leveraging the study protocol and generative AI to configure Medidata Rave EDC and Medidata eCOA systems, drastically reducing the time required to move from protocol to start-up. This delivers faster study build times, dramatically speeding time-to-market for sponsors and critical decision-making for CROs.
“Leveraging our large clinical data set from more than 38,000 trials, Medidata’s AI is redefining clinical trials, with its impact now evolving from pervasive embedding to quantifiable outcomes,” said Lisa Moneymaker, chief strategy officer, Medidata. “By prioritizing clinically-fluent, regulatory-grade AI to drive results across the trial lifecycle, we are helping our customers turn complexity into clarity and data into decisive action. Our foundational AI engine delivers platform capabilities that allow development teams to focus on resources for advancing patient care.”
To deliver truly intelligent automation, Medidata is accelerating the expansion of Dot, its core AI orchestrator that coordinates and connects the actions of domain specific AI Companions across the platform. The visual presence of Dot enables customers to instantly recognize and access the power of AI built into every step of the clinical trial process. This clear visibility accelerates the use of AI to advance new therapies to patients faster.
“As the life sciences industry increasingly moves toward embedded, enterprise AI solutions, Medidata’s AI Study Build has the potential to transform complex database build processes and accelerate market access, while Dot ensures transparency and builds trust in the use of AI,” said Dr. Nimita Limaye, Research VP, Life Sciences R&D Strategy & Technology, at IDC.
To learn more about Medidata’s AI capabilities, click here.

A report revealed a need for improved and efficient infection control and prevention systems in healthcare facilities. In 2024, Oregon recorded 1,000 hospital-acquired…