After more than 13 years at the helm of Lucasfilm, Kathleen Kennedy is stepping down from the “Star Wars” factory founded by George Lucas.
The Walt Disney Co. announced Thursday that it will now turn to Dave Filoni to steer “Star Wars”…

After more than 13 years at the helm of Lucasfilm, Kathleen Kennedy is stepping down from the “Star Wars” factory founded by George Lucas.
The Walt Disney Co. announced Thursday that it will now turn to Dave Filoni to steer “Star Wars”…
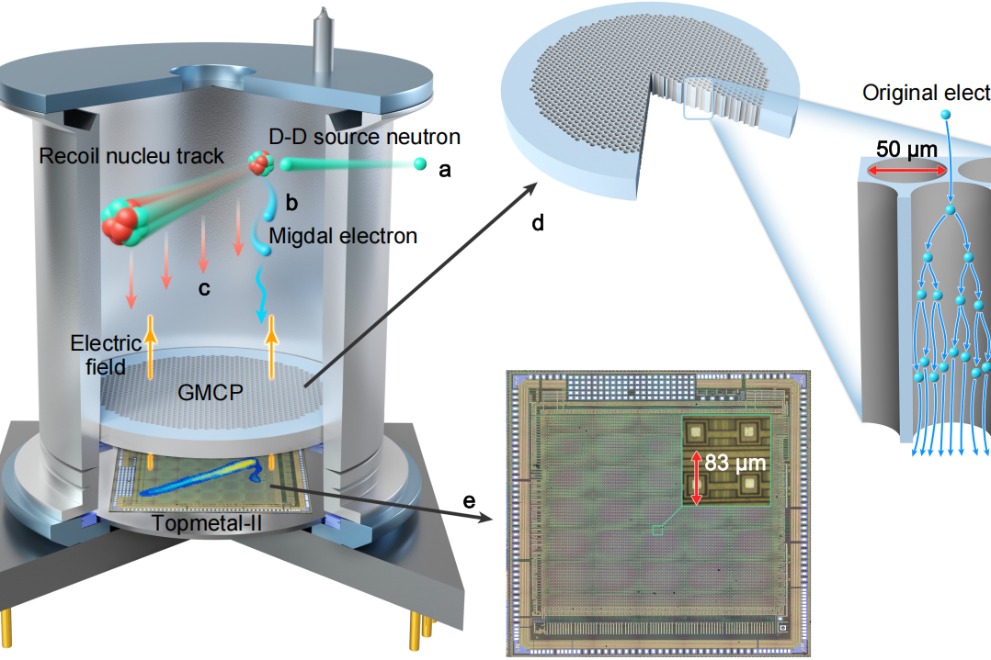
In a landmark discovery that bridges nearly a century of theoretical physics, a Chinese research team has successfully captured the first direct evidence of the Migdal effect, a breakthrough with profound implications…


.
Raimundas Karoblis, the European Union ambassador to Pakistan. Photo: APP
OpenAI Group PBC today launched ChatGPT Translate, a free translation service hosted on a standalone web page.
The rollout wasn’t accompanied by an announcement, which hints that the service may be a prototype. In July 2024, OpenAI

Vice Minister of the International Department of China’s Communist Party Sun Haiyan calls on Prime Minister Shehbaz Sharif in Islamabad. Photo: PPI


SHANGHAI and HONG KONG, Jan. 15, 2026 /PRNewswire/ — Antengene Corporation Limited (“Antengene“, SEHK: 6996.HK), a leading innovative, commercial-stage global biotech company dedicated to discovering, developing and commercializing first-in-class and/or best-in-class medicines for autoimmune disease, solid tumors and hematological malignancies indications, announced that it recently presented at the 44th Annual J.P. Morgan Healthcare Conference held in San Francisco. At the conference, Antengene shared the latest data and clinical development plans for its core clinical asset, ATG-022 (a CLDN18.2 antibody-drug conjugate [ADC]), as well as R&D progress on ATG-125 (a B7-H3 x PD-L1 bispecific ADC), its steric hindrance masking AnTenGager™ T cell engager (TCE) platform, and other key preclinical programs.
1. Core Clinical Program: ATG-022
2. Next-Generation ADCs and Proprietary TCEs
ATG-125 (B7-H3 × PD-L1 bispecific ADC): ATG-125 is an “IO+ADC ” dual-function molecule targeting B7-H3 and PD-L1, integrating the direct cytotoxic activity of an ADC with the durable immune activation of immuno-oncology (IO) therapies. By simultaneously blocking B7-H3- and PD-L1-mediated immunosuppressive signaling, ATG-125 effectively activates T cells and induces immunological memory. Preclinical studies demonstrate that the bispecific ADC delivers superior in vivo efficacy compared with single-target B7-H3-ADC or PD-L1-ADC approaches. The Company plans to submit an IND for ATG-125 in Q1 2027.
TCE platform with steric hindrance masking technology: AnTenGager™ is Antengene’s proprietary, second-generation T cell engager (TCE) platform featuring “2+1” bivalent binding for low-expressing targets, steric hindrance masking, and proprietary CD3 sequences with fast on/off kinetics to minimize cytokine release syndrome (CRS) and enhance efficacy. These characteristics support the platform’s broad applicability across autoimmune diseases, solid tumors and hematological malignancies indications. Leveraging this platform, Antengene has discovered multiple investigational programs:
3. Innovative Treatment for Autoimmune Diseases: Globally First-in-Class ATG-207
ATG-207 is a globally first-in-class dual-function biologic being developed for the treatment of T cell–mediated autoimmune diseases. The Company plans to present the preclinical data for ATG-207 for the first time at an international scientific conference in 2026.
About Antengene
Antengene Corporation Limited (“Antengene“, SEHK: 6996.HK) is a global, R&D-driven, commercial-stage biotech company focused on developing first-in-class/best-in-class therapeutics for diseases with significant unmet medical needs. Its pipeline spans from preclinical to commercial stages and includes several in-house discovered programs, including ATG-022 (CLDN18.2 ADC) , ATG-037 (oral CD73 inhibitor) , ATG-101 (PD-L1 × 4-1BB bispecific antibody) , ATG-031 (CD24-targeting macrophage activator) , and ATG-042 (oral PRMT5-MTA inhibitor).
Antengene has also developed AnTenGager™, a proprietary T cell engager 2.0 platform featuring “2+1” bivalent binding for low-expressing targets, steric hindrance masking, and proprietary CD3 sequences with fast on/off kinetics to minimize cytokine release syndrome (CRS) and enhance efficacy. These characteristics support the platform’s broad applicability across autoimmune disease, solid tumors and hematological malignancies indications.
To date, Antengene has obtained 32 investigational new drug (IND) approvals in the U.S. and Asia, and submitted new drug applications (NDAs) in 11 Asia Pacific markets. Its lead commercial asset, XPOVIO® (selinexor) , is approved in Mainland of China, Taiwan China, Hong Kong China, Macau China, South Korea, Singapore, Malaysia, Thailand, Indonesia and Australia, and has been included in the national insurance schemes in five of these markets (Mainland of China, Taiwan China, Australia, South Korea and Singapore).
Forward-looking statements
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development. For a further discussion of these and other factors that could cause future results to differ materially from any forward-looking statement, please see the other risks and uncertainties described in the Company’s Annual Report for the year ended December 31, 2024, and the documents subsequently submitted to the Hong Kong Stock Exchange.
For more information, please contact:
Investor Contacts:
Donald Lung
E-mail: [email protected]
BD Contacts:
Ariel Guo
E-mail: [email protected]
SOURCE Antengene Corporation Limited

A security vulnerability was discovered in the popular All in One SEO (AIOSEO) WordPress plugin that made it possible for low-privileged users to access a site’s global AI access token, potentially allowing them to misuse the plugin’s…