Maintaining a bedroom temperature of 24°C at night while sleeping reduces stress responses in older adults, according to new Griffith University research.
Dr Fergus O’Connor from Griffith’s School of Allied Health,…

Maintaining a bedroom temperature of 24°C at night while sleeping reduces stress responses in older adults, according to new Griffith University research.
Dr Fergus O’Connor from Griffith’s School of Allied Health,…

By Blaine Marchand
There is a popular saying that every family has a story. And for this we should be thankful that Ottawa writer (and a former long-term resident of Kitchissippi), Barbara Sibbald, skillfully recaptures for us her…
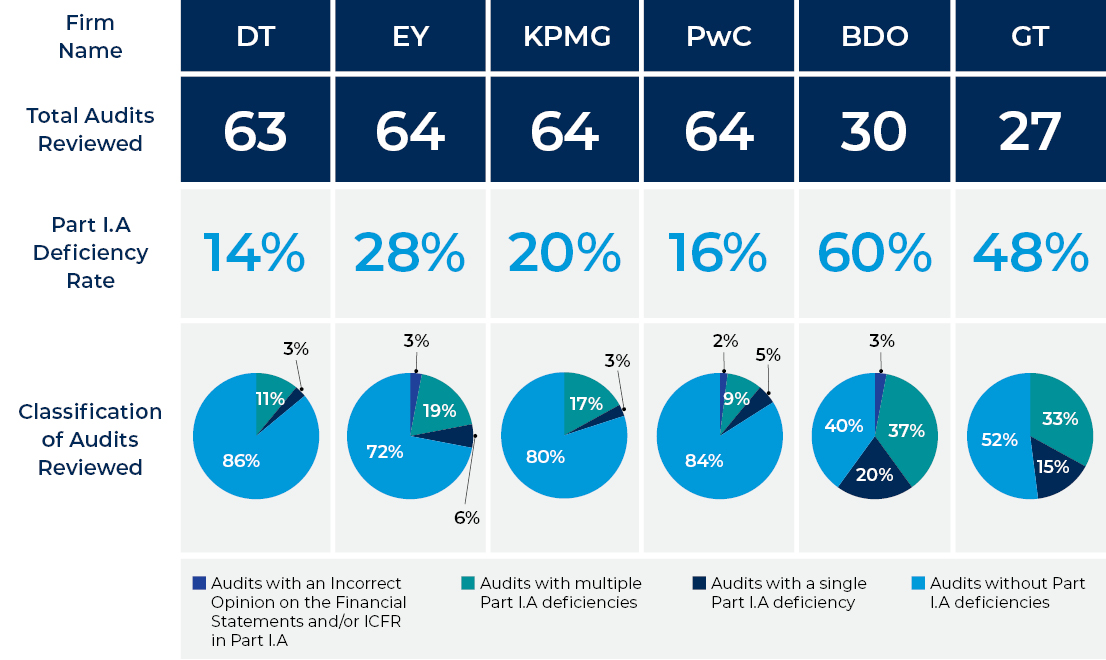
These graphs are based on inspection results included in the public portion of PCAOB inspection reports on the six largest U.S. annual firms: Deloitte & Touche LLP (DT); Ernst & Young LLP (EY); KPMG LLP (KPMG); PricewaterhouseCoopers LLP (PwC); BDO USA, P.C. (BDO); and Grant Thornton LLP (GT).
The “annual” category includes firms that have more than 100 issuer clients for which they provide audit reports (firms that the Board inspects every year as long as the firm continues to provide audit reports for more than 100 issuers each year).
The inspection data below is readily ascertainable from the public portion of the inspection report, but the data should be understood in light of contextualizing information provided with each report, including (1) it relates only to the audits selected for review, which does not constitute a representative sample of the firm’s total population of issuer audits, and only to the particular portions of the issuer audits reviewed; (2) for various reasons, inspection results are not necessarily comparable over time or among firms; (3) inspection results are not an assessment of all of the firm’s audit work nor of all of the audit procedures performed for the audits reviewed; and (4) inspection reports are not intended to serve as overall rating tools.
An audit deficiency is cited and described in Part I.A of an inspection report if it is of such significance that we believe the firm, at the time it issued its audit report(s), had not obtained sufficient appropriate audit evidence to support its opinion(s) on the issuer’s financial statements and/or internal control over financial reporting (ICFR).
Percentage of issuer audits reviewed that have at least one Part I.A deficiency in the inspection report for all six firms.
Percentage of issuer audits reviewed, by selection method, that have at least one Part I.A deficiency in the inspection report for all firms.
Number of issuer audits, by type of opinions, affected by the identified Part I.A deficiency(ies).
In our inspection reports, we classify each issuer audit reviewed in one of the categories shown in the graph based on the Part I.A deficiency or deficiencies identified in our review. The purpose of this classification system is to group and present issuer audits by the number of Part I.A deficiencies we identified within the audit as well as to highlight audits with an incorrect opinion on the financial statements and/or ICFR when applicable.
We select both integrated audits of financial statements and ICFR and audits of financial statement only.
We use a combination of risk-based and random methods to select issuer audits for review. Because our inspection process evolves over time, it can, and often does, focus on a different mix of issuer audits and audit areas from year to year and firm to firm. In addition, we utilize a target team of inspectors to perform inspection procedures in areas of current risk and emerging topics. More information on the focus of the target team procedures in each year can be found in the relevant inspection report.
These are the audit areas we have selected most frequently for review across all six firms and the number of issuer audits by focus area with and without Part I.A deficiencies. For the issuer audits selected for review, we selected these areas because they were generally significant to the issuer’s financial statements, may have included complex issues for auditors, and/or involved complex judgments in (1) estimating and auditing the reported value of related accounts and disclosures and (2) implementing and auditing the related controls.
This graph reflects the auditing standards most frequently referenced in Part I.A by audit area in the most recent inspection year, with the corresponding results for the other two years presented.
This graph depicts the number of issuer audits reviewed, by each industry sector with and without Part I.A deficiencies for all six firms. We select issuer audits for review in sectors and specific industries experiencing particularly significant disruptions or financial reporting risks.
This graph depicts the number of issuer audits reviewed, by the issuer’s revenue range, with and without Part I.A deficiencies for all six firms.
The percentage in this graph represents the number of issuer audits reviewed for that tenure range with at least one Part I.A deficiency divided by total issuer audits reviewed for that tenure range. This information is for all six firms.
The percentage in this graph represents the number of issuer audits reviewed for that partner tenure with at least one Part I.A deficiency divided by total issuer audits reviewed for that partner tenure. This information is for all six firms.
This graph depicts the number of issuer audits identified for each firm by Part I.B deficiency type. Part I.B deficiencies are certain deficiencies that relate to instances of non-compliance with PCAOB standards or rules other than those where the firm had not obtained sufficient appropriate audit evidence to support its opinions.

Launched VIZZ™ (aceclidine ophthalmic solution) 1.44% in October 2025 for the treatment of presbyopia, with broad product availability in mid-November 2025
Achieved approximately $1.6 million in net product revenue with over 20,000 prescriptions filled in Q4 2025
Over 6,500 unique ECPs prescribed VIZZ; more than 55% have prescribed multiple times in Q4 2025
SAN DIEGO, Jan. 07, 2026 (GLOBE NEWSWIRE) — LENZ Therapeutics, Inc. (Nasdaq: LENZ or “LENZ” or the “Company”), a pharmaceutical company focused on the commercialization of VIZZ™ (aceclidine ophthalmic solution) 1.44%, the first and only aceclidine-based eye drop for the treatment of presbyopia, today reported certain preliminary unaudited financial results for the fourth quarter ended December 31, 2025 and recent corporate updates.
“We are proud of the strong execution delivered in our first quarter of launch, as the team established a solid foundation of awareness, confidence, and willingness to prescribe VIZZ across the eye care professional community,” said Eef Schimmelpennink, President and Chief Executive Officer of LENZ Therapeutics. “More than 6,500 eye care professionals have already written a prescription for VIZZ, the majority of whom prescribed multiple times, signaling early confidence in VIZZ as a convenient and effective alternative to reading glasses. At the same time, over 20,000 prescriptions were filled during our first quarter of launch, exceeding our expectations and reinforcing the early momentum behind VIZZ. Building on this progress, and together with our campaign spokesperson Sarah Jessica Parker, we look forward to launching the VIZZ DTC campaign this quarter.”
Fourth Quarter 2025 Commercial Highlights
Additional Recent Corporate Updates
About LENZ Therapeutics
LENZ Therapeutics is a pharmaceutical company focused on the commercialization of VIZZTM (aceclidine ophthalmic solution) 1.44%, the first and only FDA-approved aceclidine-based eye drop for the treatment of presbyopia, a condition impacting an estimated 1.8 billion people globally and 128 million people in the United States. LENZ is commercializing VIZZ in the United States and continues to establish licensing partnerships internationally to provide access to VIZZ globally. LENZ is headquartered in San Diego, California. For more information, visit www.VIZZ.com and www.LENZ-tx.com.
About Presbyopia
Presbyopia is the inevitable loss of near vision associated with aging, impacting the daily lives of nearly all people over the age of 45. As people age, the crystalline lens in their eyes gradually hardens and becomes less able to change shape. This loss of elasticity of the lens reduces the ability of the lens to focus incoming light from near objects onto the retina. Adults over 50 years of age lose, on average, 1.5 lines of near vision every six years. Although the progression of presbyopia is gradual, presbyopes often experience an abrupt change in their daily life as the symptoms become more pronounced starting in their mid-40s, when reading glasses or other corrective aids are suddenly necessary to read text or conduct close-up work. Presbyopia is typically self-diagnosed and self-managed with over-the-counter reading glasses, or managed, after evaluation by an ECP, with prescription reading or bifocal glasses or multifocal contact lenses.
About VIZZ (aceclidine ophthalmic solution) 1.44%
VIZZ (aceclidine ophthalmic solution) 1.44% is a once-daily eye drop developed to restore clear near vision for up to 10 hours. Aceclidine is the sole active ingredient in VIZZ and provides rapid and durable near vision improvement. VIZZ is preservative-free and provided in single-dose vials. VIZZ is a predominantly pupil selective miotic that interacts with the iris with minimal ciliary muscle stimulation. VIZZ causes contraction of the iris sphincter muscle, resulting in a pinhole effect that extends depth of focus to improve vision. For more information, please visit www.VIZZ.com.
VIZZ Indication and Important Safety Information
INDICATION
VIZZ (aceclidine ophthalmic solution) 1.44% is a prescription eye drop used to treat age-related blurry near vision (presbyopia) in adults.
IMPORTANT SAFETY INFORMATION
ADVERSE REACTIONS
The most common reported adverse reactions of participants were instillation site irritation (20%), dim vision (16%), and headache (13%). Adverse reactions reported in >5% of participants were conjunctival hyperemia (8%) and ocular hyperemia (7%). The majority of adverse reactions were mild, transient, and self-resolving.
For additional information, please see the full Prescribing Information available at www.VIZZ.com/full-prescribing-information.pdf.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of federal securities laws. You can identify forward-looking statements by words such as “may,” “will,” “could,” “can,” “would,” “should,” “expect,” “intend,” “plan,” “anticipate,” “believe,” “estimate,” “predict,” “project,” “potential,” “poised,” “continue,” “ongoing” or the negative of these terms or other comparable terminology, but not all forward-looking statements will contain these words. ” Forward-looking statements in this press release include statements regarding the timing and availability of VIZZ, including the VIZZ DTC campaign; potential market size for VIZZ; its ability to meet patient needs and become standard of care; LENZ commercialization plans, including international partnering plans, and the quotations of LENZ management. These statements are based on numerous assumptions concerning VIZZ, target markets and involve substantial risks, uncertainties and other factors that may cause actual results, levels of activity, performance or achievement to be materially different from the information expressed or implied by these forward-looking statements, including those risk factors described in the section titled “Risk Factors” in our Quarterly Report on Form 10-Q filed for the quarter ended September 30, 2025 and our subsequent filings with the SEC. The unaudited results in this press release, including Q4 2025 net product revenue, are preliminary and subject to the completion of accounting and annual audit procedures and are therefore subject to adjustment. We cannot assure you that the forward-looking statements in this press release or the assumptions upon which they are based will prove to be accurate. The forward-looking statements in this press release are as of the date of this press release. Except as otherwise required by applicable law, LENZ disclaims any duty to update any forward-looking statements. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this press release.
Contact:
Dan Chevallard
LENZ Therapeutics
IR@LENZ-Tx.com
Source: LENZ Therapeutics, Inc.
Released January 7, 2026

Dark Matter (DM) remains one of the most daunting mysteries for astronomers, astrophysicists, and cosmologists. Six decades ago, the theory that the Universe was filled with mass that did not interact with normal matter in visible light…

The S&P 500 has ended lower, pulled down by declines in JPMorgan, Blackstone and other financials, while Nvidia and Alphabet lifted the Nasdaq as investors shifted towards AI-related stocks.
The drop in the S&P 500 followed an intraday record high earlier in the session on Wednesday.
Shares of housing acquisition companies tumbled after President Donald Trump said he was moving to ban Wall Street investors from buying single-family homes in a bid to reduce home prices. Real estate platform Zillow moved higher.
The Dow Jones Industrial Average fell 472.60 points, or 0.96 per cent, to 48,999.39. Data on Wednesday showed US job openings fell more than expected in November after rising marginally in October, while a separate ADP report showed that private payrolls increased less than expected in December.
While the latest labour market datasets mark a return to the standard release of economic data disrupted by the US government shutdown, they did little to change expectations of interest rate cuts from the Federal Reserve ahead of Friday’s key government payrolls report.
Investors were also monitoring geopolitical developments after the US said it seized a Russian-flagged, Venezuela-linked tanker as part of Trump’s aggressive push to dictate oil flows in the Americas and force Caracas’ socialist government to become its ally.
Major defence groups Northrop Grumman and Lockheed Martin lost ground after Trump said he would not permit dividends or stock buybacks for defence companies until they fixed problems with the production of military equipment.
Nvidia, Microsoft and Alphabet rose as investors shifted back into AI stocks following recent worries they were overvalued. Underscoring investor appetite for heavyweight AI players, Anthropic is planning a multibillion-dollar fundraising that would value the Claude chatbot maker at $US350 billion.
That would make the privately held company more valuable than the vast majority of corporations, including Advanced Micro Devices, Chevron and Wells Fargo.

LOS ANGELES (CNS) — Television personality Spencer Pratt, who lost his home in the Palisades Fire and has been a vocal critic in the months since of the city’s and state’s preparation for and response to the conflagration, announced Wednesday…


Steven McIntoshand
Lola Schroer
 BBC
BBCThe identity of the “secret…
OXFORD, Miss. – When Randle House joined the Fiesta Sports Foundation volunteer team in 2011, he dreamed of one day seeing the Ole Miss Rebels take the field for the foundation’s game, the Fiesta Bowl. Now that dream is becoming a reality.