Forty percent of consumers have used AI for shopping, and a majority plan to use it this holiday season to uncover deals
SAN JOSE, Calif., Oct. 22, 2025 /PRNewswire/ — PayPal today released its 2025 Holiday Shopping Survey, highlighting how U.S. shoppers are approaching this holiday season and what merchants need to know to capture sales and build loyalty.
“Shoppers are moving fluidly across channels, discovering products through AI, returning to stores, and choosing flexible payment options like Buy Now, Pay Later (BNPL) to maximize this holiday season,” said Michelle Gill, General Manager of Small Business and Financial Services at PayPal. “Merchants that enable seamless experiences and deliver value at every touchpoint will not only thrive this holiday season but build stronger, long-term customer loyalty.”
AI is Reshaping Holiday Shopping: Merchants Should Take Note
This holiday season, shoppers are turning to AI to find inspiration and make purchasing decisions. Merchants that ensure their products are visible and optimized across AI-powered platforms will be best positioned to capture demand and drive sales in this AI-driven shopping season.
- AI is becoming a go-to shopping tool, with 40% of Americans – led by 61% of Gen Z and 57% of Millennials – having used AI to assist with a purchase in the past year, and one in five doing so regularly.
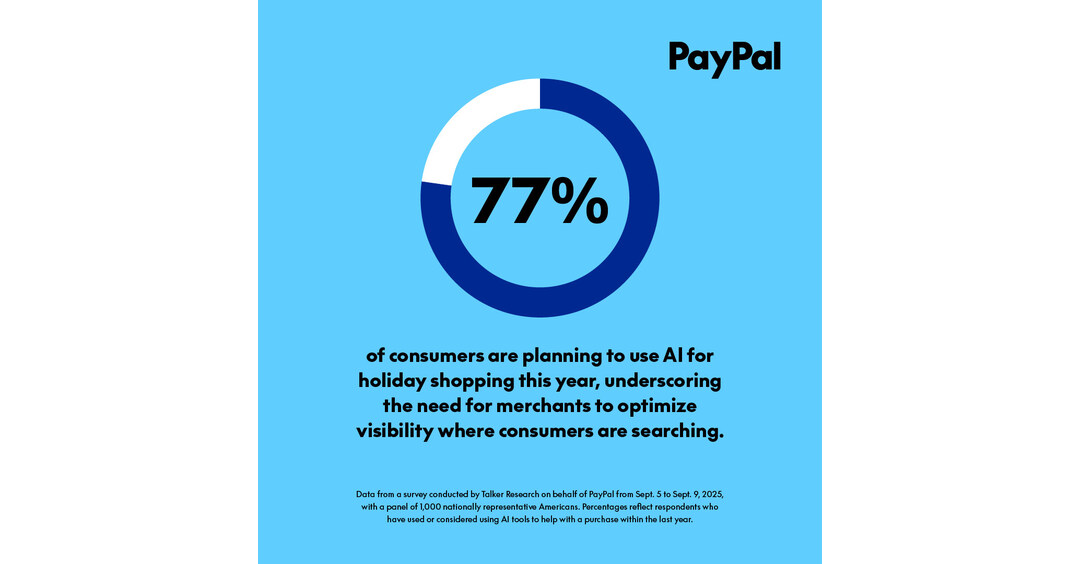
- Adoption is accelerating, as 77% of past or potential AI shoppers plan to use it as a shopping assistant this holiday season, underscoring the need for merchants to appear where consumers are searching.
- Holiday shoppers are turning to AI for value and guidance, with the top ways they plan to use it including finding the best deals (34%), comparing products (30%), and discovering gift ideas or recommendations (26%).
As AI reshapes how consumers shop, flexible payment options like BNPL are transforming how they pay, creating a smoother path from discovery to purchase for both shoppers and merchants.
BNPL Helps Shoppers Manage Cash Flow While Boosting Merchant Conversion
Once considered an alternative payment method, BNPL is now a mainstream expectation at checkout. BNPL has become a vital tool for managing cash flow and helping consumers shop with confidence and flexibility – while empowering merchants to increase conversion and average order value.
- Half of consumers plan to use BNPL as a flexible payment option for holiday shopping this year, citing affordability and budget control as the top reasons.
- BNPL is driving purchasing decisions, with 52% of shoppers saying they’re more likely to make a purchase when BNPL is available as a payment option.
- Gen Z and Millennials lead BNPL adoption as one in four use it regularly, and another third have tried it at least once, making BNPL a core part of how younger generations prefer to pay.
“Merchants can enhance the shopper experience and boost sales by making flexible payment options like BNPL visible throughout the shopping journey,” continued Gill. “When shoppers know they can pay overtime, they’re more likely to complete their purchase. PayPal data shows offering BNPL leads to a 91% higher average order value for enterprises and 62% higher for small businesses, meaning it isn’t just a nice-to-have, it’s a proven advantage to win.”
Omnichannel Returns as Shoppers Expect Seamless, Rewarding Experiences Everywhere
Shoppers are taking more paths to purchase this holiday season than ever before, signaling the true return of omnichannel retail. Consumers expect seamless experiences, meaningful rewards, and value – and merchants that connect every channel will capture both sales and long-term customer relationships.
- In-store shopping is resurging, as 64% of shoppers plan to shop in-store this holiday season, with 41% planning to shop both online and in-store.
- Online shopping still matters, with 28% planning to shop primarily online, underscoring that no single channel dominates the holiday experience.
- Rewards drive decisions where Americans shop as 74% are more likely to shop with merchants offering cash back or rewards.
Success this holiday season will hinge on connection, not just convenience. Merchants that unify rewards, online and in-store experiences will deepen loyalty and help fuel long-term growth. To help with this, PayPal is expanding Pay Monthly to in-store purchases across the U.S., and shoppers can also earn 5% cash back on all PayPal BNPL purchases, in-store and online, throughout the holiday season.
About the 2025 Holiday Shopping Survey
PayPal commissioned Talker Research to survey 1,000 nationally representative U.S. adults online from September 5-9, 2025. Percentages for AI data reflect respondents who have used or considered using AI tools to help with a purchase within the last year. Percentages for BNPL data reflect respondents who have ever used or considered using BNPL to make a purchase.
About PayPal
PayPal has been revolutionizing commerce globally for more than 25 years. Creating innovative experiences that make moving money, selling, and shopping simple, personalized, and secure, PayPal empowers consumers and businesses in approximately 200 markets to join and thrive in the global economy. For more information, visit https://www.paypal.com, https://about.pypl.com, and https://investor.pypl.com.
Media Contact:
PayPal Media Relations
press@paypal.com


SOURCE PayPal Holdings, Inc.