- Global Airlines Outlook Neutral Amid Resilient Demand Fitch Ratings
- Aviation Flies Into 2026 With Good Prospects Despite Challenges Energy Intelligence
- CITIC Securities Aviation 2026 Investment Strategy: Focus on Airlines’ Profit Inflection Points; Reconstruction of Prosperity Cycle May Be Imminent 富途牛牛
Category: 3. Business
-
Global Airlines Outlook Neutral Amid Resilient Demand – Fitch Ratings
-

EU fines Elon Musk’s X 120 million euros for breaching bloc’s social media law
LONDON (AP) — European Union regulators on Friday fined X, Elon Musk’s social media platform, 120 million euros ($140 million) for breaches of the bloc’s digital regulations, in a move that risks rekindling tensions with Washington over free speech.
The European Commission issued its decision following an investigation it opened two years ago into X under the 27-nation bloc’s Digital Services Act, also known as the DSA.
It’s the first time that the EU has issued a so-called non-compliance decision since rolling out the DSA. The sweeping rulebook requires platforms to take more responsibility for protecting European users and cleaning up harmful or illegal content and products on their sites, under threat of hefty fines.
READ MORE: France will investigate Musk’s Grok after AI chatbot posted Holocaust denial claims
The Commission, the bloc’s executive arm, said it was punishing X because of three different breaches of the DSA’s transparency requirements. The decision could rile President Donald Trump, whose administration has lashed out at digital regulations, complained that Brussels was targeting U.S. tech companies and vowed to retaliate.
U.S. Secretary of State Marco Rubio posted on his X account that the Commission’s fine was akin to an attack on the American people. Musk later agreed with Rubio’s sentiment.
“The European Commission’s $140 million fine isn’t just an attack on @X, it’s an attack on all American tech platforms and the American people by foreign governments,” Rubio wrote. “The days of censoring Americans online are over.”
Vice President JD Vance, posting on X ahead of the decision, accused the Commission of seeking to fine X “for not engaging in censorship.”
“The EU should be supporting free speech not attacking American companies over garbage,” he wrote.
Officials denied the rules were intended to muzzle Big Tech companies. The Commission is “not targeting anyone, not targeting any company, not targeting any jurisdictions based on their color or their country of origin,” spokesman Thomas Regnier told a regular briefing in Brussels. “Absolutely not. This is based on a process, democratic process.”
X did not respond immediately to an email request for comment.
EU regulators had already outlined their accusations in mid-2024 when they released preliminary findings of their investigation into X.
Regulators said X’s blue checkmarks broke the rules because on “deceptive design practices” and could expose users to scams and manipulation.
Before Musk acquired X, when it was previously known as Twitter, the checkmarks mirrored verification badges common on social media and were largely reserved for celebrities, politicians and other influential accounts, such as Beyonce, Pope Francis, writer Neil Gaiman and rapper Lil Nas X.
After he bought it in 2022, the site started issuing the badges to anyone who wanted to pay $8 per month.
That means X does not meaningfully verify who’s behind the account, “making it difficult for users to judge the authenticity of accounts and content they engage with,” the Commission said in its announcement.
X also fell short of the transparency requirements for its ad database, regulators said.
Platforms in the EU are required to provide a database of all the digital advertisements they have carried, with details such as who paid for them and the intended audience, to help researches detect scams, fake ads and coordinated influence campaigns. But X’s database, the Commission said, is undermined by design features and access barriers such as “excessive delays in processing.”
Regulators also said X also puts up “unnecessary barriers” for researchers trying to access public data, which stymies research into systemic risks that European users face.
“Deceiving users with blue checkmarks, obscuring information on ads and shutting out researchers have no place online in the EU. The DSA protects users,” Henna Virkkunen, the EU’s executive vice-president for tech sovereignty, security and democracy, said in a prepared statement.
The Commission also wrapped up a separate DSA case Friday involving TikTok’s ad database after the video-sharing platform promised to make changes to ensure full transparency.
AP Writer Lorne Cook in Brussels contributed to this report.
A free press is a cornerstone of a healthy democracy.
Support trusted journalism and civil dialogue.
Continue Reading
-

Treasury market logs worst weekly rout since April, in a bad sign for borrowers
By Vivien Lou Chen
Instead of falling as Wall Street braces for another Fed rate cut next week, the 10-year Treasury yield rose 12 basis points for the week to almost 4.14%, the most since April
Recent U.S. economic data has bond-market participants uncertain about the Federal Reserve’s ability to cut interest rates next year.
In a bad sign for anyone looking for a reprieve from higher borrowing costs, longer-dated U.S. government debt sold off sharply this week.
The 10-year note and 30-year bond had their worst weekly performances since April and May on conflicting economic data that is sowing doubts about how much the Federal Reserve can cut interest rates in 2026.
Traders widely expect a quarter-point rate cut next week that will push the central bank’s main policy target down to between 3.5% and 3.75%. But they were less certain about next year and see a decent chance that the central bank won’t cut rates again through next March.
Benchmark yields are significant to households, businesses and governments because they influence the cost of borrowing on everything from mortgages, auto loans and credit cards to capital projects, while also affecting interest payments on the national debt.
On Friday, the 10-year Treasury yield BX:TMUBMUSD10Y rose 12 basis points for the week to almost 4.14%, the most for any week since April, according to Dow Jones Market Data. The yield on the 30-year bond BX:TMUBMUSD30Y advanced by a similar amount to almost 4.8%, the biggest weekly increase since May. Yields move in the opposite direction to prices, so a rise in these rates is a reflection of the selloffs that took place in the underlying government maturities.
“Yields are heading back to the higher end of the range that we’ve seen since summer,” said Tom Nakamura, a currency strategist and co-head of fixed income at AGF Investments in Toronto, which had almost $43.6 billion (C$60.4 billion) in assets under management and fee-earning assets as of November.
“One of the things driving this is some of the economic data, like jobless claims and University of Michigan consumer sentiment, which are showing resilience and may curtail the Fed from easing much further,” he said.
Data released on Thursday showed initial jobless claims fell to a more than three-year low of 191,000 in the week that ended Nov. 29. On Friday, the University of Michigan’s consumer-sentiment reading inched up by 2.3 points to 53.3 for December, and the rate of U.S. inflation based on the personal consumption expenditures index for September remained stable.
On a more downbeat note, however, payroll processor ADP reported that privately run businesses eliminated 32,000 jobs in November, for the biggest decline since the spring of 2023.
Meanwhile, bond traders are keeping an eye on rising Japanese yields and the possibility of a rate hike by the Bank of Japan later this month. Concerns are that Japan’s bond-market developments, triggered by worries over economic-stimulus efforts under Prime Minister Sanae Takaichi, might lead to higher yields in the U.S.
Read: Investors are worrying about potential spillover from surging Japanese bond yields. Here’s a breakdown of what matters.
“Globally, we’re seeing some pressure on bonds from fiscal policy – for example, in Japan, with yields on Japanese government bonds rising because the country’s fiscal policy is seen as adding to inflation concerns,” Nakamura said via phone. “The market will tend to focus on fiscal concerns in waves. And as they get highlighted in one market, this tends to shine a light on others as well, particularly in countries where fiscal policy has been more stimulative, such as the U.S.”
On Friday, 1- through 30-year yields finished broadly higher. All three major U.S. stock indexes ended in the green, with the S&P 500 SPX and Nasdaq COMP each securing a fourth day of gains.
-Vivien Lou Chen
This content was created by MarketWatch, which is operated by Dow Jones & Co. MarketWatch is published independently from Dow Jones Newswires and The Wall Street Journal.
(END) Dow Jones Newswires
12-05-25 1626ET
Copyright (c) 2025 Dow Jones & Company, Inc.
Continue Reading
-

Noskova volunteers at Zanzibar school, calls experience ‘unforgettable’
While many players headed to the beach for some much-needed rest and relaxation after the grueling season, Linda Noskova opted to go in a different direction with her time off.
The 21-year-old Czech headed to Zanzibar, Tanzania and spent an eye-opening week volunteering at a local school. Noskova experienced what life is like for the 300 students firsthand, living in a volunteer house with shared rooms and no air conditioning.
The trip offered a great deal of perspective, and it was one of the most rewarding experiences of Noskova’s life.
“Traveling to Zanzibar, Africa to volunteer was one of the most meaningful experiences of my life, and finally making that long-time dream happen felt both surreal and unforgettable,” the World No. 13 said. “I prepared myself for the cultural shock, but nothing could compare to being there in person — seeing how people live, understanding their challenges and feeling their warmth despite having so little.”
Noskova was touched and inspired by how kind and appreciative the students were, and is determined to continue providing opportunities for the community.
“Their kindness, their excitement and their resilience changed something in me,” Noskova said, “and supporting them now feels like the most natural thing to do. This trip opened my eyes in ways I didn’t expect, and it reminded me how fortunate we are — an experience I believe everyone should have at least once in their life.”
Noskova’s volunteer work comes on the heels of the season of her career. She made her debut in the Top 20 in October following her run to the final of the WTA 1000 event in Beijing, where she lost to Amanda Anisimova in three sets. Earlier in the year she made the final in Prague, and reached another final in her last tournament of the year, in Tokyo.
Continue Reading
-
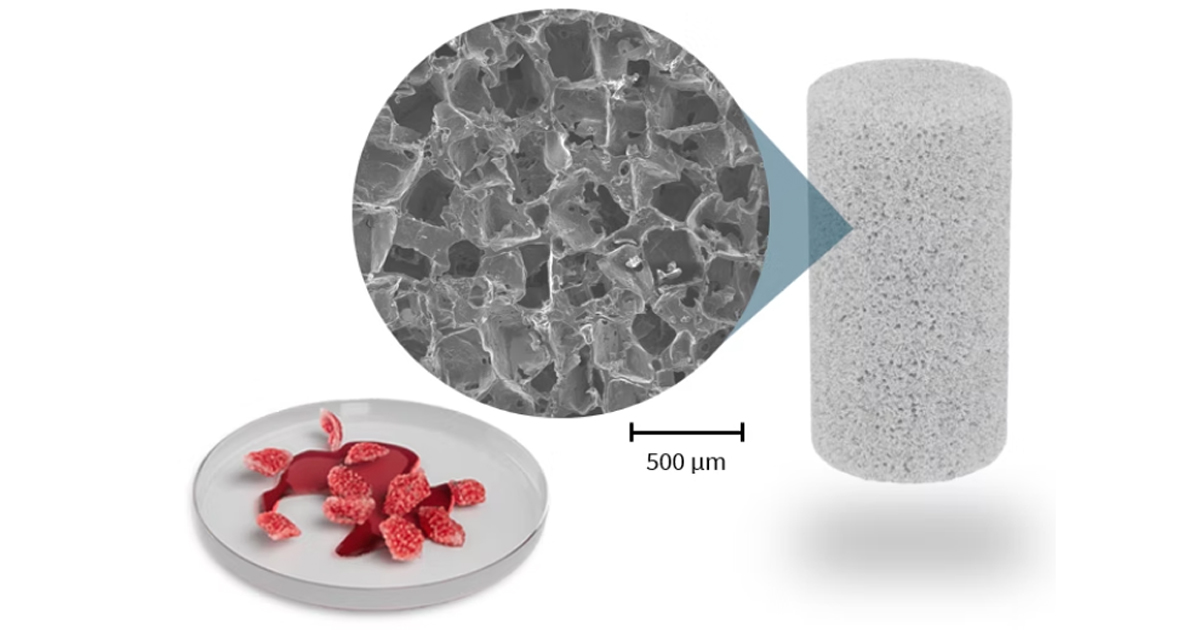
Bone Regeneration Technology Used in First Patient Surgery | News
A bone regeneration device using bioactive materials pioneered by Querrey Simpson Institute for Regenerative Engineering at Northwestern University (QSI RENU) director Guillermo Ameer was recently used in its first surgical case, Acuitive Technologies Inc. announced.

Called Citregraft, the technology is composed of the citrate-based polymers developed in the Ameer laboratory as well as bioactive glass. It is a highly porous, synthetic bone graft substitute that can be morselized to fit irregular defects. After placement in a bony void, Citregraft binds local growth factors and steadily releases citrate to support bone regeneration before resorbing and being replaced by the patient’s natural bone.
In its first surgical use, the sponge-like material repaired bone tissue that was harvested as part of an ACL reconstruction on the knee. The device received FDA clearance last November and is one of several products in Acuitive Technologies’ CITREGEN product line.
“Twenty years after the first report of citrate-based polymers we now see another major milestone: the use of our biomaterial technology to develop a citrate-based bioactive scaffold to regenerate bone tissue in patients,” said Ameer, the Daniel Hale Williams Professor of Biomedical Engineering at Northwestern Engineering and a professor of surgery in Northwestern’s Feinberg School of Medicine. “This builds on the success and expands the impact our biomaterial technology and collaboration with Acuitive Technologies has made on medical devices that regenerate musculoskeletal tissues.
“I am very proud of our research team members, past and present, who have contributed to the development and applications of citrate-based biomaterials. I am humbled to see that the impact of our publications goes beyond academic research, being validated by industry, and now improving the lives of patients.”
Editor’s note: Intellectual property associated with the Citregraft is subject to an exclusive license between Northwestern and Vesseltek Biomedical. Pursuant to the terms of that license, Northwestern has financial interest in Vesseltek Biomedical. Vesseltek Biomedical sublicenses Northwestern intellectual property associated with Citregraft to Acuitive Technologies. Ameer has financial interest in Vesseltek Biomedical and serves on the scientific advisory board of Acuitive Technologies.
Continue Reading
-

Johnson & Johnson’s INLEXZO™ (gemcitabine intravesical system) delivers 74 percent disease-free survival at one year in BCG-unresponsive, high-risk, papillary-only NMIBC
New data from Cohort 4 of the SunRISe-1 study show more than 95 percent of patients remained progression free at one year, with more than 92 percent not undergoing bladder removal
Patients with this type of bladder cancer have limited choices beyond radical cystectomy, highlighting the need for newer therapies for bladder preservation
RARITAN, N.J., Dec. 5, 2025 /PRNewswire/ — Johnson & Johnson (NYSE:JNJ) announced today that new data from the investigational Cohort 4 of the Phase 2b SunRISe-1 study show treatment with gemcitabine intravesical system resulted in high one-year disease-free survival (DFS), progression-free survival (PFS), and overall survival (OS) rates in patients with Bacillus Calmette-Guérin (BCG)-unresponsive, high-risk, papillary-only non-muscle invasive bladder cancer (NMIBC).1 These data were featured as a late-breaking oral presentation at the Society of Urologic Oncology (SUO) 2025 Annual Meeting and build upon data presented at the 2025 American Urological Association (AUA) Annual Meeting.
“The findings are meaningful, as the majority of patients remained free of cancer recurrence at one year despite having papillary tumors that carry a high risk for recurrence and a significant risk of progression to a more aggressive, muscle-invasive stage of disease,” said Siamak Daneshmand*, M.D., Professor of Urology, University of Southern California, and presenting author. “Bladder removal has traditionally been the primary path forward for these patients, a life-altering procedure that can have a significant impact on a patient’s quality of life.”
“At Johnson & Johnson, we are committed to developing innovative treatments for patients with high-risk NMIBC who have few options beyond life-altering surgery,” said Christopher Cutie, M.D., Vice President, Disease Area Leader, Bladder Cancer, Johnson & Johnson Innovative Medicine. “Those with papillary-only disease face particularly difficult decisions, as surgical removal of the bladder has long been the standard of care for patients who are unresponsive or resistant to BCG.”
Cohort 4 of the Phase 2b SunRISe-1 study focused on 52 patients with papillary-only, high-risk NMIBC whose disease did not respond or stopped responding to BCG therapy and who were ineligible for or declined radical cystectomy. The therapy was administered every three weeks for six months, followed by every 12 weeks for up to an additional 18 months, to evaluate its potential to prevent the recurrence or progression of high-grade papillary tumors.1 The results support continued evaluation in the ongoing Phase 3 SunRISe-5 study (NCT06211764) comparing gemcitabine intravesical system to chemotherapy in patients with previously BCG-treated, papillary-only NMIBC.
At median follow-up of 15.9 months (range, 4-20 months), the one-year DFS rate was 74.3 percent (95 percent confidence interval [CI], 59.2-84.6), meaning nearly three out of four patients remained free from cancer recurrence. Results were similar across patients with high-grade Ta and T1 papillary tumors, 74.8 percent and 74.1 percent, respectively (95 percent CI, 54.3-87.1 and 48.5-88.3). At one year, PFS was 95.6 percent (95 percent CI, 83.5-98.9) and OS was 98 percent (95 percent CI, 86.6-99.7). Notably, 92.3 percent of patients did not undergo radical cystectomy, and median time to cystectomy was not reached. Overall Health Status and Physical Functioning scores were maintained during treatment with gemcitabine intravesical system.1
The therapy was generally well-tolerated. Most patients (80.8 percent) experienced treatment-related side effects that were low grade, such as mild urinary symptoms, including burning, frequency, or urgency. More serious side effects (13.5 percent) were uncommon and most often involved bladder pain. A small number of patients (7.7 percent) discontinued treatment due to side effects, and no treatment-related deaths were reported.1
About SunRISe-1, Cohort 4
SunRISe-1 (NCT04640623) is an ongoing Phase 2b, open-label, multicenter study evaluating the efficacy and safety of gemcitabine intravesical system in patients with BCG-unresponsive HR-NMIBC who are ineligible for, or elected not to undergo, radical cystectomy. Cohort 4 specifically enrolls patients with papillary-only disease. The primary endpoint of Cohort 4 is disease-free survival (DFS) rate at 12 months. Key secondary endpoints included safety and tolerability.2
About High-Risk Non-Muscle Invasive Bladder Cancer
High-risk non-muscle invasive bladder cancer is a type of non-invasive bladder cancer that is more likely to recur or spread beyond the lining of the bladder, called the urothelium, and progress to muscle invasive bladder cancer compared to low-risk NMIBC.3,4 HR-NMIBC makes up 15-44 percent of patients with NMIBC and is characterized by a high-grade, large tumor size, presence of multiple tumors, and carcinoma in situ.5 Radical cystectomy is currently recommended for HR-NMIBC patients who fail BCG therapy, with over 90 percent cancer-specific survival if performed before muscle-invasive progression.6,7 Given that NMIBC typically affects older patients, many may be unwilling or unfit to undergo radical cystectomy.8 The high rates of recurrence and progression can pose significant morbidity and distress for these patients.3,4
About INLEXZO™ (gemcitabine intravesical system)
INLEXZO™ is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.
The safety and efficacy of INLEXZO™ is being evaluated in clinical trials in patients with MIBC in SunRISe-4, and HR-NMIBC in SunRISe-1, SunRISe-3, and SunRISe-5.
The legal manufacturer for INLEXZO™ is Janssen Biotech, Inc.
INLEXZO™ IMPORTANT SAFETY INFORMATION9
CONTRAINDICATIONS
INLEXZO™ is contraindicated in patients with:
- Perforation of the bladder.
- Prior hypersensitivity reactions to gemcitabine or any component of the product.
WARNINGS AND PRECAUTIONS
Risks in Patients with Perforated Bladder
INLEXZO™ may lead to systemic exposure to gemcitabine and to severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised.
Evaluate the bladder before the intravesical administration of INLEXZO™ and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored.
Risk of Metastatic Bladder Cancer with Delayed Cystectomy
Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. The risk of developing muscle invasive or metastatic bladder cancer increases the longer cystectomy is delayed in the presence of persisting CIS.
Of the 83 evaluable patients with BCG-unresponsive CIS treated with INLEXZO™ in Cohort 2 of SunRISe-1, 7 patients (8%) progressed to muscle invasive (T2 or greater) bladder cancer. Three patients (3.5%) had progression determined at the time of cystectomy. The median time between determination of persistent or recurrent CIS or T1 and progression to muscle invasive disease was 94 days.
Magnetic Resonance Imaging (MRI) Safety
INLEXZO™ can only be safely scanned with MRI under certain conditions. Refer to section 5.3 of the USPI for details on conditions.
Embryo-Fetal Toxicity
Based on animal data and its mechanism of action, INLEXZO™ can cause fetal harm when administered to a pregnant woman if systemic exposure occurs. In animal reproduction studies, systemic administration of gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after final removal of INLEXZO™. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after final removal of INLEXZO™.
ADVERSE REACTIONS
Serious adverse reactions occurred in 24% of patients receiving INLEXZO™. Serious adverse reactions that occurred in >2% of patients included urinary tract infection, hematuria, pneumonia, and urinary tract pain. Fatal adverse reactions occurred in 1.2% of patients who received INLEXZO™, including cognitive disorder.
The most common (>15%) adverse reactions, including laboratory abnormalities, were urinary frequency, urinary tract infection, dysuria, micturition urgency, decreased hemoglobin, increased lipase, urinary tract pain, decreased lymphocytes, hematuria, increased creatinine, increased potassium, increased AST, decreased sodium, bladder irritation, and increased ALT.
USE IN SPECIFIC POPULATIONS
Pregnancy
There are no available data on the use of INLEXZO™ in pregnant women to inform a drug-associated risk. Please see Embryo-Fetal Toxicity for risk information related to pregnancy.
Lactation
Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment and for 1 week after final removal of INLEXZO™.
Females and Males of Reproductive Potential
Pregnancy Testing – Verify pregnancy status in females of reproductive potential prior to initiating INLEXZO™.
Contraception – Please see Embryo-Fetal Toxicity for information regarding contraception.
Infertility (Males) – Based on animal studies, INLEXZO™ may impair fertility in males of reproductive potential. It is not known whether these effects on fertility are reversible.
Geriatric Use
Of the patients given INLEXZO™ monotherapy in Cohort 2 of SunRISe-1, 72% were 65 years of age or older and 34% were 75 years or older. There were insufficient numbers of patients <65 years of age to determine if these patients respond differently to patients 65 years of age and older.
Please read full Prescribing Information and Instructions for Use for INLEXZO™.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at https://www.jnj.com/ or at www.innovativemedicine.jnj.com. Follow us at @JNJInnovMed.Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding product development and the potential benefits and treatment impact of INLEXZO™. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at http://www.sec.gov, http://www.jnj.com, or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.*Dr. Siamak Daneshmand has provided consulting, advisory, and speaking services to Johnson & Johnson; he has not been paid for any media work.
1 Daneshmand, S., & colleagues. (2025). Gemcitabine intravesical system (TAR-200) monotherapy in patients with Bacillus Calmette-Guérin–unresponsive papillary disease–only high-risk non–muscle-invasive bladder cancer: 1-year disease-free survival results from SUNRISE-1. Abstract presented at the Society of Urologic Oncology (SUO) Annual Meeting.
2 ClinicalTrials.gov. A Study of TAR-200 in Combination With Cetrelimab, TAR-200 Alone, or Cetrelimab Alone in Participants With Non-Muscle Invasive Bladder Cancer (NMIBC) Unresponsive to Intravesical Bacillus Calmette-Guérin Who Are Ineligible for or Elected Not to Undergo Radical Cystectomy (SunRISe-1). https://clinicaltrials.gov/study/NCT04640623. Accessed December 2025.
3 Grab-Heyne K, Henne C, Mariappan P, et al. Intermediate and high-risk non–muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023;13:1170124.
4 Lieblich A, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. The management of non–muscle-invasive bladder cancer: a comparison of European and UK guidelines. J Clin Urol. 2018;11(2):144-148.
5 Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75-94. doi:10.1016/j.eururo.2021.08.010
6 Brooks NA, O’Donnell MA. Treatment options in non–muscle-invasive bladder cancer after BCG failure. Indian J Urol. 2015;31(4):312-319. doi:10.4103/0970-1591.166475
7 Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939-949.
8 Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer. 2013;119(17):3219-3227.
9 INLEXZO™ U.S. Prescribing Information.SOURCE Johnson & Johnson
Continue Reading
-
Johnson & Johnson’s INLEXZO™ (gemcitabine intravesical system) delivers 74 percent disease-free survival at one year in BCG-unresponsive, high-risk, papillary-only NMIBC
RARITAN, New Jersey, December 5, 2025 – Johnson & Johnson (NYSE:JNJ) announced today that new data from the investigational Cohort 4 of the Phase 2b SunRISe-1 study show treatment with gemcitabine intravesical system resulted in high one-year disease-free survival (DFS), progression-free survival (PFS), and overall survival (OS) rates in patients with Bacillus Calmette-Guérin (BCG)-unresponsive, high-risk, papillary-only non-muscle invasive bladder cancer (NMIBC).1 These data were featured as a late-breaking oral presentation at the Society of Urologic Oncology (SUO) 2025 Annual Meeting and build upon data presented at the 2025 American Urological Association (AUA) Annual Meeting.
“The findings are meaningful, as the majority of patients remained free of cancer recurrence at one year despite having papillary tumors that carry a high risk for recurrence and a significant risk of progression to a more aggressive, muscle-invasive stage of disease,” said Siamak Daneshmand*, M.D., Professor of Urology, University of Southern California, and presenting author. “Bladder removal has traditionally been the primary path forward for these patients, a life-altering procedure that can have a significant impact on a patient’s quality of life.”
“At Johnson & Johnson, we are committed to developing innovative treatments for patients with high-risk NMIBC who have few options beyond life-altering surgery,” said Christopher Cutie, M.D., Vice President, Disease Area Leader, Bladder Cancer, Johnson & Johnson Innovative Medicine. “Those with papillary-only disease face particularly difficult decisions, as surgical removal of the bladder has long been the standard of care for patients who are unresponsive or resistant to BCG.”
Cohort 4 of the Phase 2b SunRISe-1 study focused on 52 patients with papillary-only, high-risk NMIBC whose disease did not respond or stopped responding to BCG therapy and who were ineligible for or declined radical cystectomy. The therapy was administered every three weeks for six months, followed by every 12 weeks for up to an additional 18 months, to evaluate its potential to prevent the recurrence or progression of high-grade papillary tumors.1 The results support continued evaluation in the ongoing Phase 3 SunRISe-5 study (NCT06211764) comparing gemcitabine intravesical system to chemotherapy in patients with previously BCG-treated, papillary-only NMIBC.
At median follow-up of 15.9 months (range, 4-20 months), the one-year DFS rate was 74.3 percent (95 percent confidence interval [CI], 59.2-84.6), meaning nearly three out of four patients remained free from cancer recurrence. Results were similar across patients with high-grade Ta and T1 papillary tumors, 74.8 percent and 74.1 percent, respectively (95 percent CI, 54.3-87.1 and 48.5-88.3). At one year, PFS was 95.6 percent (95 percent CI, 83.5-98.9) and OS was 98 percent (95 percent CI, 86.6-99.7). Notably, 92.3 percent of patients did not undergo radical cystectomy, and median time to cystectomy was not reached. Overall Health Status and Physical Functioning scores were maintained during treatment with gemcitabine intravesical system.1
The therapy was generally well-tolerated. Most patients (80.8 percent) experienced treatment-related side effects that were low grade, such as mild urinary symptoms, including burning, frequency, or urgency. More serious side effects (13.5 percent) were uncommon and most often involved bladder pain. A small number of patients (7.7 percent) discontinued treatment due to side effects, and no treatment-related deaths were reported.1
About SunRISe-1, Cohort 4
SunRISe-1 (NCT04640623) is an ongoing Phase 2b, open-label, multicenter study evaluating the efficacy and safety of gemcitabine intravesical system in patients with BCG-unresponsive HR-NMIBC who are ineligible for, or elected not to undergo, radical cystectomy. Cohort 4 specifically enrolls patients with papillary-only disease. The primary endpoint of Cohort 4 is disease-free survival (DFS) rate at 12 months. Key secondary endpoints included safety and tolerability.2About High-Risk Non-Muscle Invasive Bladder Cancer
High-risk non-muscle invasive bladder cancer is a type of non-invasive bladder cancer that is more likely to recur or spread beyond the lining of the bladder, called the urothelium, and progress to muscle invasive bladder cancer compared to low-risk NMIBC.3,4 HR-NMIBC makes up 15-44 percent of patients with NMIBC and is characterized by a high-grade, large tumor size, presence of multiple tumors, and carcinoma in situ.5 Radical cystectomy is currently recommended for HR-NMIBC patients who fail BCG therapy, with over 90 percent cancer-specific survival if performed before muscle-invasive progression.6,7 Given that NMIBC typically affects older patients, many may be unwilling or unfit to undergo radical cystectomy.8 The high rates of recurrence and progression can pose significant morbidity and distress for these patients.3,4About INLEXZO™ (gemcitabine intravesical system)
INLEXZO™ is approved by the U.S. Food and Drug Administration (FDA) for the treatment of adult patients with Bacillus Calmette-Guérin (BCG)-unresponsive, non-muscle invasive bladder cancer (NMIBC) with carcinoma in situ (CIS), with or without papillary tumors.The safety and efficacy of INLEXZO™ is being evaluated in clinical trials in patients with MIBC in SunRISe-4, and HR-NMIBC in SunRISe-1, SunRISe-3, and SunRISe-5.
The legal manufacturer for INLEXZO™ is Janssen Biotech, Inc.
INLEXZO™ IMPORTANT SAFETY INFORMATION9
CONTRAINDICATIONS
INLEXZO™ is contraindicated in patients with:
- Perforation of the bladder.
- Prior hypersensitivity reactions to gemcitabine or any component of the product.
WARNINGS AND PRECAUTIONS
Risks in Patients with Perforated Bladder
INLEXZO™ may lead to systemic exposure to gemcitabine and to severe adverse reactions if administered to patients with a perforated bladder or to those in whom the integrity of the bladder mucosa has been compromised.
Evaluate the bladder before the intravesical administration of INLEXZO™ and do not administer to patients with a perforated bladder or mucosal compromise until bladder integrity has been restored.
Risk of Metastatic Bladder Cancer with Delayed Cystectomy
Delaying cystectomy in patients with BCG-unresponsive CIS could lead to development of muscle invasive or metastatic bladder cancer, which can be lethal. The risk of developing muscle invasive or metastatic bladder cancer increases the longer cystectomy is delayed in the presence of persisting CIS.
Of the 83 evaluable patients with BCG-unresponsive CIS treated with INLEXZO™ in Cohort 2 of SunRISe-1, 7 patients (8%) progressed to muscle invasive (T2 or greater) bladder cancer. Three patients (3.5%) had progression determined at the time of cystectomy. The median time between determination of persistent or recurrent CIS or T1 and progression to muscle invasive disease was 94 days.
Magnetic Resonance Imaging (MRI) Safety
INLEXZO™ can only be safely scanned with MRI under certain conditions. Refer to section 5.3 of the USPI for details on conditions.
Embryo-Fetal Toxicity
Based on animal data and its mechanism of action, INLEXZO™ can cause fetal harm when administered to a pregnant woman if systemic exposure occurs. In animal reproduction studies, systemic administration of gemcitabine was teratogenic, embryotoxic, and fetotoxic in mice and rabbits.
Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after final removal of INLEXZO™. Advise male patients with female partners of reproductive potential to use effective contraception during treatment and for 3 months after final removal of INLEXZO™.
ADVERSE REACTIONS
Serious adverse reactions occurred in 24% of patients receiving INLEXZO™. Serious adverse reactions that occurred in >2% of patients included urinary tract infection, hematuria, pneumonia, and urinary tract pain. Fatal adverse reactions occurred in 1.2% of patients who received INLEXZO™, including cognitive disorder.
The most common (>15%) adverse reactions, including laboratory abnormalities, were urinary frequency, urinary tract infection, dysuria, micturition urgency, decreased hemoglobin, increased lipase, urinary tract pain, decreased lymphocytes, hematuria, increased creatinine, increased potassium, increased AST, decreased sodium, bladder irritation, and increased ALT.
USE IN SPECIFIC POPULATIONS
Pregnancy
There are no available data on the use of INLEXZO™ in pregnant women to inform a drug-associated risk.
Please see Embryo-Fetal Toxicity for risk information related to pregnancy.Lactation
Because of the potential for serious adverse reactions in breastfed infants, advise women not to breastfeed during treatment and for 1 week after final removal of INLEXZO™.
Females and Males of Reproductive Potential
Pregnancy Testing – Verify pregnancy status in females of reproductive potential prior to initiating INLEXZO™.Contraception – Please see Embryo-Fetal Toxicity for information regarding contraception.
Infertility (Males) – Based on animal studies, INLEXZO™ may impair fertility in males of reproductive potential. It is not known whether these effects on fertility are reversible.
Geriatric Use
Of the patients given INLEXZO™ monotherapy in Cohort 2 of SunRISe-1, 72% were 65 years of age or older and 34% were 75 years or older. There were insufficient numbers of patients <65 years of age to determine if these patients respond differently to patients 65 years of age and older.
Please read full Prescribing Information and Instructions for Use for INLEXZO™.
About Johnson & Johnson
At Johnson & Johnson, we believe health is everything. Our strength in healthcare innovation empowers us to build a world where complex diseases are prevented, treated, and cured, where treatments are smarter and less invasive, and solutions are personal. Through our expertise in Innovative Medicine and MedTech, we are uniquely positioned to innovate across the full spectrum of healthcare solutions today to deliver the breakthroughs of tomorrow, and profoundly impact health for humanity. Learn more at https://www.jnj.com/ or at www.innovativemedicine.jnj.com. Follow us at @JNJInnovMed.Cautions Concerning Forward-Looking Statements
This press release contains “forward-looking statements” as defined in the Private Securities Litigation Reform Act of 1995 regarding product development and the potential benefits and treatment impact of INLEXZO™. The reader is cautioned not to rely on these forward-looking statements. These statements are based on current expectations of future events. If underlying assumptions prove inaccurate or known or unknown risks or uncertainties materialize, actual results could vary materially from the expectations and projections of Johnson & Johnson. Risks and uncertainties include, but are not limited to: challenges and uncertainties inherent in product research and development, including the uncertainty of clinical success and of obtaining regulatory approvals; uncertainty of commercial success; manufacturing difficulties and delays; competition, including technological advances, new products and patents attained by competitors; challenges to patents; product efficacy or safety concerns resulting in product recalls or regulatory action; changes in behavior and spending patterns of purchasers of health care products and services; changes to applicable laws and regulations, including global health care reforms; and trends toward health care cost containment. A further list and descriptions of these risks, uncertainties and other factors can be found in Johnson & Johnson’s most recent Annual Report on Form 10-K, including in the sections captioned “Cautionary Note Regarding Forward-Looking Statements” and “Item 1A. Risk Factors,” and in Johnson & Johnson’s subsequent Quarterly Reports on Form 10-Q and other filings with the Securities and Exchange Commission. Copies of these filings are available online at http://www.sec.gov, http://www.jnj.com, or on request from Johnson & Johnson. Johnson & Johnson does not undertake to update any forward-looking statement as a result of new information or future events or developments.Footnotes
*Dr. Siamak Daneshmand has provided consulting, advisory, and speaking services to Johnson & Johnson; he has not been paid for any media work.1 Daneshmand, S., & colleagues. (2025). Gemcitabine intravesical system (TAR-200) monotherapy in patients with Bacillus Calmette-Guérin–unresponsive papillary disease–only high-risk non–muscle-invasive bladder cancer: 1-year disease-free survival results from SUNRISE-1. Abstract presented at the Society of Urologic Oncology (SUO) Annual Meeting.
2 ClinicalTrials.gov. A Study of TAR-200 in Combination With Cetrelimab, TAR-200 Alone, or Cetrelimab Alone in Participants With Non-Muscle Invasive Bladder Cancer (NMIBC) Unresponsive to Intravesical Bacillus Calmette-Guérin Who Are Ineligible for or Elected Not to Undergo Radical Cystectomy (SunRISe-1). https://clinicaltrials.gov/study/NCT04640623. Accessed December 2025.
3 Grab-Heyne K, Henne C, Mariappan P, et al. Intermediate and high-risk non–muscle-invasive bladder cancer: an overview of epidemiology, burden, and unmet needs. Front Oncol. 2023;13:1170124.
4 Lieblich A, Henne C, Mariappan P, Geiges G, Pöhlmann J, Pollock RF. The management of non–muscle-invasive bladder cancer: a comparison of European and UK guidelines. J Clin Urol. 2018;11(2):144-148.
5 Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on Non-muscle-invasive Bladder Cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75-94. doi:10.1016/j.eururo.2021.08.010
6 Brooks NA, O’Donnell MA. Treatment options in non–muscle-invasive bladder cancer after BCG failure. Indian J Urol. 2015;31(4):312-319. doi:10.4103/0970-1591.166475
7 Guancial EA, Roussel B, Bergsma DP, et al. Bladder cancer in the elderly patient: challenges and solutions. Clin Interv Aging. 2015;10:939-949.
8 Chamie K, Litwin MS, Bassett JC, et al. Recurrence of high-risk bladder cancer: A population-based analysis. Cancer. 2013;119(17):3219-3227.
9 INLEXZO™ U.S. Prescribing Information.
Continue Reading
-
Copper Rises to Record on Bullish Citi Outlook, Supply Concerns – Bloomberg.com
- Copper Rises to Record on Bullish Citi Outlook, Supply Concerns Bloomberg.com
- Copper eases from all-time peak Business Recorder
- Copper price extends record run as Citi makes bullish call Mining.com
- AI Data Centers Could Consume Half a Million Tons of Copper Annually by 2030 U.S. Global Investors
- Copper price unlikely to stay above $11,000/t: Goldman Sachs Kallanish
Continue Reading
-

IPO Boards Need to Adjust Amid Governance Upheaval // Cooley // Global Law Firm
This content is provided for general informational purposes only, and your access or use of the content does not create an attorney-client relationship between you or your organization and Cooley LLP, Cooley (UK) LLP, or any other affiliated practice or entity (collectively referred to as “Cooley”). By accessing this content, you agree that the information provided does not constitute legal or other professional advice. This content is not a substitute for obtaining legal advice from a qualified attorney licensed in your jurisdiction, and you should not act or refrain from acting based on this content. This content may be changed without notice. It is not guaranteed to be complete, correct or up to date, and it may not reflect the most current legal developments. Prior results do not guarantee a similar outcome. Do not send any confidential information to Cooley, as we do not have any duty to keep any information you provide to us confidential. When advising companies, our attorney-client relationship is with the company, not with any individual. This content may have been generated with the assistance of artificial intelligence (Al) in accordance with our Al Principles, may be considered Attorney Advertising and is subject to our legal notices.
Continue Reading
-
ICICI Prudential Asset's India IPO to open on December 12 – Reuters
- ICICI Prudential Asset’s India IPO to open on December 12 Reuters
- ICICI Bank says ICICI Prudential AMC files RHP for IPO Business Standard
- Prudential announces filing of red herring prospectus for ICICI Prudential Asset Management IPO marketscreener.com
- RCS – Prudential PLC – Pudential plc – Filing of RHP for IPAMC IPO TradingView
- ICICI Prudential AMC sets price band of Rs 2061-2165 a share for IPO Moneycontrol
Continue Reading