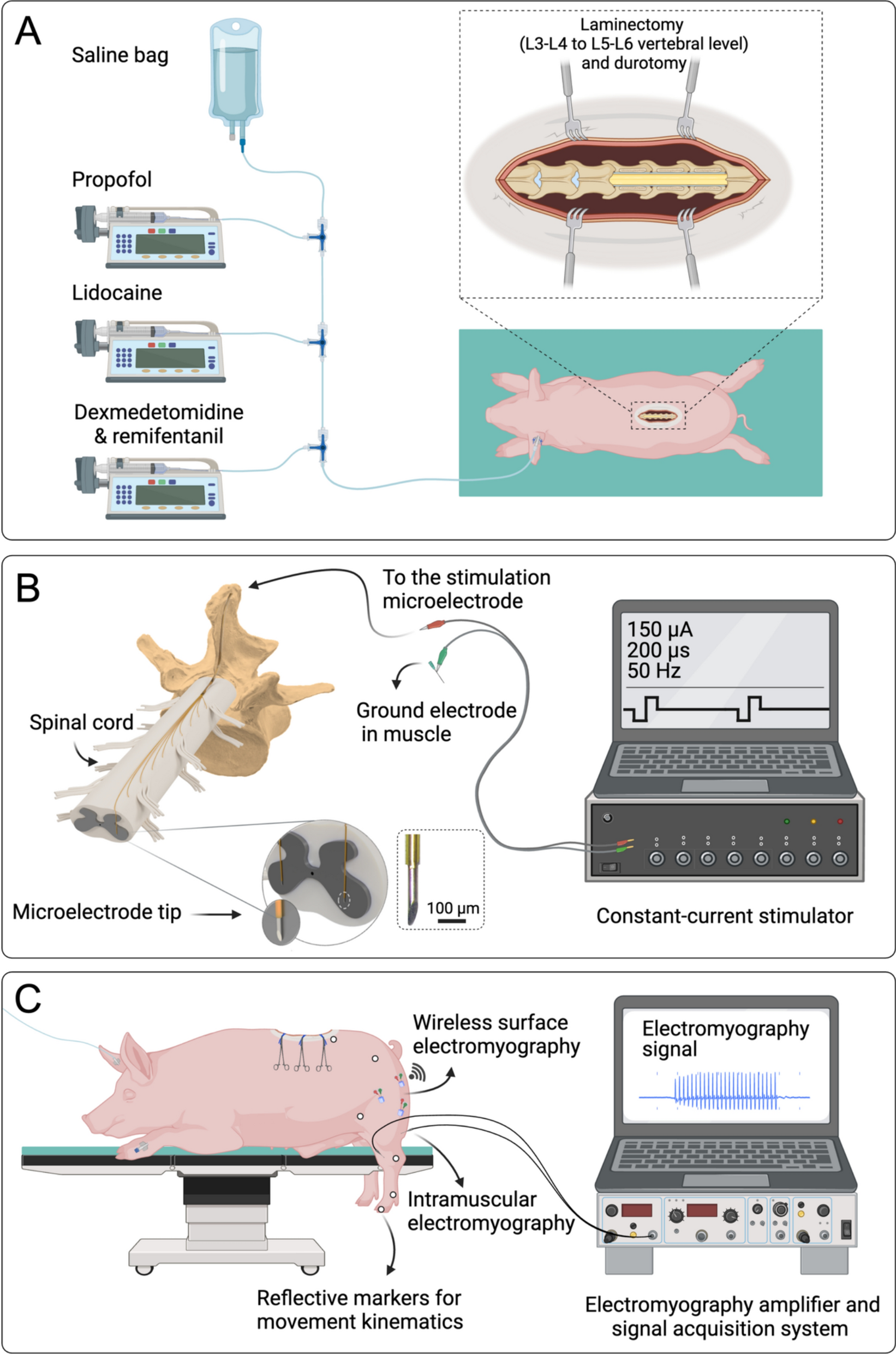
Alizadeh A, Dyck SM, Karimi-Abdolrezaee S. Traumatic spinal cord injury: an overview of pathophysiology, models and acute injury mechanisms. Front Neurol. 2019;10:282.
Google Scholar
National Spinal Cord Injury Statistical Center. Traumatic spinal cord injury facts and figures at a glance. Birmingham, AL: University of Alabama at Birmingham [Internet]. 2024; Available from: https://msktc.org/sites/default/files/Facts-and-Figures-2024-Eng-508.pdf
Anderson KD. Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma. 2004;21(10):1371–83.
Google Scholar
Brown-Triolo DL, Roach MJ, Nelson K, Triolo R. Consumer perspectives on mobility: implications for neuroprosthesis design. Journal of Rehabilitation Research & Development. 2002;39(6).
Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, et al. Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. Lancet. 2011;377(9781):1938–47.
Google Scholar
Rowald A, Komi S, Demesmaeker R, Baaklini E, Hernandez-Charpak SD, Paoles E, et al. Activity-dependent spinal cord neuromodulation rapidly restores trunk and leg motor functions after complete paralysis. Nat Med. 2022;28(2):260–71.
Google Scholar
Gill ML, Grahn PJ, Calvert JS, Linde MB, Lavrov IA, Strommen JA, et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med. 2018;24(11):1677–82.
Google Scholar
Angeli CA, Boakye M, Morton RA, Vogt J, Benton K, Chen Y, et al. Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med. 2018;379(13):1244–50.
Google Scholar
Hofstoetter US, Perret I, Bayart A, Lackner P, Binder H, Freundl B, et al. Spinal motor mapping by epidural stimulation of lumbosacral posterior roots in humans. iScience. 2021. https://doi.org/10.1016/j.isci.2020.101930.
Google Scholar
Gorgey AS, Trainer R, Sutor TW, Goldsmith JA, Alazzam A, Goetz LL, et al. A case study of percutaneous epidural stimulation to enable motor control in two men after spinal cord injury. Nat Commun. 2023;14(1):2064.
Google Scholar
Mesbah S, Herrity A, Ugiliweneza B, Angeli C, Gerasimenko Y, Boakye M, et al. Neuroanatomical mapping of the lumbosacral spinal cord in individuals with chronic spinal cord injury. Brain Commun. 2023;5(1):fcac330.
Google Scholar
Mong ER, Kethireddy S, Staudt MD. Spinal cord stimulator paddle lead revision and replacement for misplaced or displaced electrodes. World Neurosurg. 2024. https://doi.org/10.1016/j.wneu.2024.03.154.
Google Scholar
Dombovy-Johnson ML, D’Souza RS, Ha CT, Hagedorn JM. Incidence and risk factors for spinal cord stimulator lead migration with or without loss of efficacy: a retrospective review of 91 consecutive thoracic lead implants. Neuromodulation Technol Neural Interface. 2022;25(5):731–7.
Alazzam AM, Ballance WB, Smith AC, Rejc E, Weber KA, Trainer R, et al. Peak slope ratio of the recruitment curves compared to muscle evoked potentials to optimize standing configurations with percutaneous epidural stimulation after spinal cord injury. J Clin Med. 2024;13(5):1344.
Google Scholar
Boakye M, Ball T, Dietz N, Sharma M, Angeli C, Rejc E, et al. Spinal cord epidural stimulation for motor and autonomic function recovery after chronic spinal cord injury: a case series and technical note. Surg Neurol Int. 2023. https://doi.org/10.25259/SNI_1074_2022.
Google Scholar
Rejc E, Angeli CA, Bryant N, Harkema SJ. Effects of stand and step training with epidural stimulation on motor function for standing in chronic complete paraplegics. J Neurotrauma. 2017;34(9):1787–802.
Google Scholar
Capogrosso M, Wenger N, Raspopovic S, Musienko P, Beauparlant J, Luciani LB, et al. A computational model for epidural electrical stimulation of spinal sensorimotor circuits. J Neurosci. 2013;33(49):19326–40.
Google Scholar
Greiner N, Barra B, Schiavone G, Lorach H, James N, Conti S, et al. Recruitment of upper-limb motoneurons with epidural electrical stimulation of the cervical spinal cord. Nat Commun. 2021;12(1):435.
Google Scholar
Choi EH, Gattas S, Brown NJ, Hong JD, Limbo JN, Chan AY, et al. Epidural electrical stimulation for spinal cord injury. Neural Regen Res. 2021;16(12):2367–75.
Google Scholar
Saigal R, Renzi C, Mushahwar VK. Intraspinal microstimulation generates functional movements after spinal-cord injury. IEEE Trans Neural Syst Rehabil Eng. 2004;12(4):430–40.
Google Scholar
Mushahwar VK, Gillard DM, Gauthier MJ, Prochazka A. Intraspinal micro stimulation generates locomotor-like and feedback-controlled movements. IEEE Trans Neural Syst Rehabil Eng. 2002;10(1):68–81.
Google Scholar
Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT. Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. J Neural Eng. 2013;10(3):036001.
Google Scholar
Zimmermann JB, Seki K, Jackson A. Reanimating the arm and hand with intraspinal microstimulation. J Neural Eng. 2011;8(5):054001.
Google Scholar
Gaunt RA, Prochazka A, Mushahwar VK, Guevremont L, Ellaway PH. Intraspinal microstimulation excites multisegmental sensory afferents at lower stimulus levels than local α-motoneuron responses. J Neurophysiol. 2006;96(6):2995–3005.
Google Scholar
Zimmermann JB, Jackson A. Closed-loop control of spinal cord stimulation to restore hand function after paralysis. Front Neurosci. 2014;8:87.
Google Scholar
Bizzi E, Giszter SF, Loeb E, Mussa-Ivaldi FA, Saltiel P. Modular organization of motor behavior in the frog’s spinal cord. Trends Neurosci. 1995;18(10):442–6.
Google Scholar
Giszter SF. Spinal primitives and intra-spinal micro-stimulation (ISMS) based prostheses: a neurobiological perspective on the “known unknowns” in ISMS and future prospects. Front Neurosci. 2015;9:72.
Google Scholar
Moritz CT, Lucas TH, Perlmutter SI, Fetz EE. Forelimb movements and muscle responses evoked by microstimulation of cervical spinal cord in sedated monkeys. J Neurophysiol. 2007;97(1):110–20.
Google Scholar
Hachmann JT, Jeong JH, Grahn PJ, Mallory GW, Evertz LQ, Bieber AJ, et al. Large animal model for development of functional restoration paradigms using epidural and intraspinal stimulation. PLoS ONE. 2013;8(12):e81443.
Google Scholar
Sharpe AN, Jackson A. Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. J Neural Eng. 2014;11(1):016005.
Google Scholar
Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Exp Neurol. 2000;163(2):422–9.
Google Scholar
Tawakol O, Herman MD, Foxley S, Mushahwar VK, Towle VL, Troyk PR. In-vivo testing of a novel wireless intraspinal microstimulation interface for restoration of motor function following spinal cord injury. Artif Organs. 2024;48(3):263–73.
Google Scholar
Nashold B, Friedman H, Grimes J. Electrical stimulation of the conus medullaris to control bladder emptying in paraplegia: a ten-year review. Stereotact Funct Neurosurg. 1982;45(1–2):40–3.
Google Scholar
Nashold BS, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R. Electromicturition in paraplegia: implantation of a spinal neuroprosthesis. Arch Surg. 1972;104(2):195–202.
Google Scholar
Boergens KM, Tadić A, Hopper MS, McNamara I, Fell D, Sahasrabuddhe K, et al. Laser ablation of the pia mater for insertion of high-density microelectrode arrays in a translational sheep model. J Neural Eng. 2021;18(4):045008.
Rocca A, Lehner C, Wafula-Wekesa E, Luna E, Fernández-Cornejo V, Abarca-Olivas J, et al. Robot-assisted implantation of a microelectrode array in the occipital lobe as a visual prosthesis. J Neurosurg. 2023;140(4):1169–76.
Google Scholar
Roser F, Tatagiba M, Maier G. Spinal robotics: current applications and future perspectives. Neurosurgery. 2013;72:A12–8.
Zeng B, Meng F, Ding H, Wang G. A surgical robot with augmented reality visualization for stereoelectroencephalography electrode implantation. Int J Comput Assist Radiol Surg. 2017;12:1355–68.
Google Scholar
Prochazka A, Mushahwar V, Yakovenko S. Activation and coordination of spinal motoneuron pools after spinal cord injury. Prog Brain Res. 2002;137:109–24.
Google Scholar
Guevremont L, Renzi CG, Norton JA, Kowalczewski J, Saigal R, Mushahwar VK. Locomotor-related networks in the lumbosacral enlargement of the adult spinal cat: activation through intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2006;14(3):266–72.
Google Scholar
Toossi A, Bergin B, Marefatallah M, Parhizi B, Tyreman N, Everaert DG, et al. Comparative neuroanatomy of the lumbosacral spinal cord of the rat, cat, pig, monkey, and human. Sci Rep. 2021;11(1):1955.
Google Scholar
Lee JH, Jones CF, Okon EB, Anderson L, Tigchelaar S, Kooner P, et al. A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma. 2013;30(3):142–59.
Google Scholar
Leonard AV, Menendez JY, Pat BM, Hadley MN, Floyd CL. Localization of the corticospinal tract within the porcine spinal cord: implications for experimental modeling of traumatic spinal cord injury. Neurosci Lett. 2017;648:1–7.
Google Scholar
Smith AC, Ahmed RU, Weber KA, Negahdar M, Gibson D, Boakye M, et al. Spinal cord lesion MRI and behavioral outcomes in a miniature pig model of spinal cord injury: exploring preclinical potential through an ad hoc comparison with human SCI. Spinal Cord Ser Cases. 2024;10(1):44.
Google Scholar
Ahmed RU, Knibbe CA, Wilkins F, Sherwood LC, Howland DR, Boakye M. Porcine spinal cord injury model for translational research across multiple functional systems. Exp Neurol. 2023;359:114267.
Google Scholar
Mirkiani S, Roszko DA, O’Sullivan CL, Faridi P, Hu DS, Fang D, et al. Overground gait kinematics and muscle activation patterns in the Yucatan mini pig. J Neural Eng. 2022;19(2):026009.
Boakye M, Morehouse J, Ethridge J, Burke DA, Khattar NK, Kumar C, et al. Treadmill-based gait kinematics in the Yucatan mini pig. J Neurotrauma. 2020;37(21):2277–91.
Google Scholar
Schiavone G, Wagner F, Fallegger F, Kang X, Vachicouras N, Barra B, et al. Long-term functionality of a soft electrode array for epidural spinal cord stimulation in a minipig model. In IEEE; 2018. p. 1432–5.
Hogan MK, Barber SM, Rao Z, Kondiles BR, Huang M, Steele WJ, et al. A wireless spinal stimulation system for ventral activation of the rat cervical spinal cord. Sci Rep. 2021;11(1):14900.
Google Scholar
Afridi AK, Steele AG, Martin C, Sayenko DG, Barber SM. Ventral epidural stimulation for motor recovery after spinal cord injury: illustrative case. Journal of Neurosurgery: Case Lessons. 2024;8(12).
Lin D, Lee JM, Wang C, Park HG, Lieber CM. Injectable ventral spinal stimulator evokes programmable and biomimetic hindlimb motion. Nano Lett. 2023;23(13):6184–92.
Google Scholar
Harland B, Kow CY, Svirskis D. Spinal intradural electrodes: opportunities, challenges and translation to the clinic. Neural Regen Res. 2023. https://doi.org/10.4103/1673-5374.380895.
Google Scholar
Olmsted ZT, Wu PB, Katouzian A, Dorsi MJ. Intrathecal placement of percutaneous spinal cord stimulation leads: illustrative cases. Journal of Neurosurgery: Case Lessons. 2024;8(13).
Chapman KB, Sayed D, Lamer T, Hunter C, Weisbein J, Patel KV, et al. Best practices for dorsal root ganglion stimulation for chronic pain: guidelines from the American Society of Pain and Neuroscience. J Pain Res. 2023;839–79.
King KW, Cusack WF, Nanivadekar AC, Ayers CA, Urbin M, Gaunt RA, et al. DRG microstimulation evokes postural responses in awake, standing felines. J Neural Eng. 2019;17(1):016014.
Google Scholar
Ayers CA, Fisher LE, Gaunt RA, Weber DJ. Microstimulation of the lumbar DRG recruits primary afferent neurons in localized regions of lower limb. J Neurophysiol. 2016;116(1):51–60.
Google Scholar
Dalrymple AN, Ting JE, Bose R, Trevathan JK, Nieuwoudt S, Lempka SF, et al. Stimulation of the dorsal root ganglion using an Injectrode®. J Neural Eng. 2021;18(5):056068.
Toossi A, Everaert DG, Perlmutter SI, Mushahwar VK. Functional organization of motor networks in the lumbosacral spinal cord of non-human primates. Sci Rep. 2019;9(1):13539.
Google Scholar
Toossi A, Everaert DG, Seres P, Jaremko JL, Robinson K, Kao CC, et al. Ultrasound-guided spinal stereotactic system for intraspinal implants. J Neurosurg Spine. 2018;29(3):292–305.
Google Scholar
Mirkiani S, O’Sullivan CL, Roszko DA, Faridi P, Hu DS, Everaert DG, et al. Safety of mapping the motor networks in the spinal cord using penetrating microelectrodes in Yucatan minipigs. Journal of Neurosurgery: Spine. 2024;1(aop):1–13.
Borrell JA, Frost SB, Peterson J, Nudo RJ. A 3d map of the hindlimb motor representation in the lumbar spinal cord in Sprague Dawley rats. J Neural Eng. 2016;14(1):016007.
Google Scholar
Mushahwar VK, Horch KW. Selective activation of muscle groups in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Trans Rehabil Eng. 2000;8(1):11–21.
Google Scholar
Bamford JA, Todd KG, Mushahwar VK. The effects of intraspinal microstimulation on spinal cord tissue in the rat. Biomaterials. 2010;31(21):5552–63.
Google Scholar
Roszko DA, Mirkiani S, Tyreman N, Wilson D, Toossi A, Mushahwar VK. Laser-microfabricated polymer multielectrodes for intraspinal microstimulation. IEEE Trans Biomed Eng. 2022;70(1):354–65.
Google Scholar
Toossi A, Everaert DG, Uwiera RR, Hu DS, Robinson K, Gragasin FS, et al. Effect of anesthesia on motor responses evoked by spinal neural prostheses during intraoperative procedures. J Neural Eng. 2019;16(3):036003.
Google Scholar
Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol. 1997;382(1):46–76.
Google Scholar
Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Exp Brain Res. 1999;129:401–16.
Google Scholar
Capogrosso M, Milekovic T, Borton D, Wagner F, Moraud EM, Mignardot JB, et al. A brain–spine interface alleviating gait deficits after spinal cord injury in primates. Nature. 2016;539(7628):284–8.
Google Scholar
Sharrard W. The segmental innervation of the lower limb muscles in man: Arris and Gale lecture delivered at the Royal College of Surgeons of England on 2nd January 1964. Ann R Coll Surg Engl. 1964;35(2):106.
Google Scholar
Sharrard W. The distribution of the permanent paralysis in the lower limb in poliomyelitis: a clinical and pathological study. J Bone Joint Surgery British. 1955;37(4):540–58.
Yakovenko S, Mushahwar V, VanderHorst V, Holstege G, Prochazka A. Spatiotemporal activation of lumbosacral motoneurons in the locomotor step cycle. J Neurophysiol. 2002;87(3):1542–53.
Google Scholar
Bamford J, Putman C, Mushahwar V. Intraspinal microstimulation preferentially recruits fatigue-resistant muscle fibres and generates gradual force in rat. J Physiol. 2005;569(3):873–84.
Google Scholar
Holinski B, Mazurek K, Everaert D, Toossi A, Lucas-Osma A, Troyk P, et al. Intraspinal microstimulation produces over-ground walking in anesthetized cats. J Neural Eng. 2016;13(5):056016.
Google Scholar
Snow S, Horch KW, Mushahwar VK. Intraspinal microstimulation using cylindrical multielectrodes. IEEE Trans Biomed Eng. 2006;53(2):311–9.
Google Scholar
Lau B, Guevremont L, Mushahwar VK. Strategies for generating prolonged functional standing using intramuscular stimulation or intraspinal microstimulation. IEEE Trans Neural Syst Rehabil Eng. 2007;15(2):273–85.
Google Scholar
Vogelstein RJ, Tenore FV, Guevremont L, Etienne-Cummings R, Mushahwar VK. A silicon central pattern generator controls locomotion in vivo. IEEE Trans Biomed Circuits Syst. 2008;2(3):212–22.
Google Scholar
Dalrymple AN, Roszko DA, Sutton RS, Mushahwar VK. Pavlovian control of intraspinal microstimulation to produce over-ground walking. J Neural Eng. 2020;17(3):036002.
Google Scholar
Dalrymple AN, Everaert DG, Hu DS, Mushahwar VK. A speed-adaptive intraspinal microstimulation controller to restore weight-bearing stepping in a spinal cord hemisection model. J Neural Eng. 2018;15(5):056023.
Google Scholar
Fiori L, Castiglia SF, Chini G, Draicchio F, Sacco F, Serrao M, et al. The lower limb muscle co-activation map during human locomotion: from slow walking to running. Bioengineering. 2024;11(3):288.
Google Scholar
Cheng R, Sui Y, Sayenko D, Burdick JW. Motor control after human SCI through activation of muscle synergies under spinal cord stimulation. IEEE Trans Neural Syst Rehabil Eng. 2019;27(6):1331–40.
Google Scholar
Rasmussen S, Chan A, Goslow G Jr. The cat step cycle: electromyographic patterns for hindlimb muscles during posture and unrestrained locomotion. J Morphol. 1978;155(3):253–69.
Google Scholar
Aagaard P, Simonsen E, Andersen J, Magnusson S, Bojsen-Møller F, Dyhre-Poulsen P. Antagonist muscle coactivation during isokinetic knee extension. Scand J Med Sci Sports. 2000;10(2):58–67.
Google Scholar
Latash ML. Muscle coactivation: definitions, mechanisms, and functions. J Neurophysiol. 2018;120(1):88–104.
Google Scholar
Baratta R, Solomonow M, Zhou B, Letson D, Chuinard R, D’ambrosia R. Muscular coactivation: the role of the antagonist musculature in maintaining knee stability. Am J Sports Med. 1988;16(2):113–22.
Google Scholar
Tsuchida T, Ensini M, Morton S, Baldassare M, Edlund T, Jessell T, et al. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79(6):957–70.
Google Scholar
Tripodi M, Stepien AE, Arber S. Motor antagonism exposed by spatial segregation and timing of neurogenesis. Nature. 2011;479(7371):61–6.
Google Scholar
Takeoka A, Arber S. Functional local proprioceptive feedback circuits initiate and maintain locomotor recovery after spinal cord injury. Cell Rep. 2019;27(1):71–85.
Google Scholar
Ronzano R, Skarlatou S, Barriga BK, Bannatyne BA, Bhumbra GS, Foster JD, et al. Spinal premotor interneurons controlling antagonistic muscles are spatially intermingled. Elife. 2022;11:e81976.
Google Scholar
Levine AJ, Hinckley CA, Hilde KL, Driscoll SP, Poon TH, Montgomery JM, et al. Identification of a cellular node for motor control pathways. Nat Neurosci. 2014;17(4):586–93.
Google Scholar
Hanson TL, Diaz-Botia CA, Kharazia V, Maharbiz MM, Sabes PN. The “sewing machine” for minimally invasive neural recording. BioRxiv. 2019;578542.
Vomero M, Ciarpella F, Zucchini E, Kirsch M, Fadiga L, Stieglitz T, et al. On the longevity of flexible neural interfaces: establishing biostability of polyimide-based intracortical implants. Biomaterials. 2022;281:121372.
Google Scholar
Luan L, Wei X, Zhao Z, Siegel JJ, Potnis O, Tuppen CA, et al. Ultraflexible nanoelectronic probes form reliable, glial scar–free neural integration. Sci Adv. 2017;3(2):e1601966.
Google Scholar
Lee Y, Shin H, Lee D, Choi S, Cho I, Seo J. A lubricated nonimmunogenic neural probe for acute insertion trauma minimization and long-term signal recording. Adv Sci. 2021;8(15):2100231.
Google Scholar
Sridar S, Churchward MA, Mushahwar VK, Todd KG, Elias AL. Peptide modification of polyimide-insulated microwires: towards improved biocompatibility through reduced glial scarring. Acta Biomater. 2017;60:154–66.
Google Scholar
Toossi A, Everaert DG, Azar A, Dennison CR, Mushahwar VK. Mechanically stable intraspinal microstimulation implants for human translation. Ann Biomed Eng. 2017;45:681–94.
Google Scholar
Soloukey S, de Rooij JD, Osterthun R, Drenthen J, De Zeeuw CI, Huygen FJ, et al. The dorsal root ganglion as a novel neuromodulatory target to evoke strong and reproducible motor responses in chronic motor complete spinal cord injury: a case series of five patients. Neuromodul Technol Neural Interface. 2021;24(4):779–93.
Holinski BJ, Mazurek KA, Everaert DG, Stein RB, Mushahwar VK. Restoring stepping after spinal cord injury using intraspinal microstimulation and novel control strategies. In IEEE; 2011. p. 5798–801.
Kramer J, Liem L, Russo M, Smet I, Van Buyten JP, Huygen F. Lack of body positional effects on paresthesias when stimulating the dorsal root ganglion (DRG) in the treatment of chronic pain. Neuromodul Technol Neural Interface. 2015;18(1):50–7.
Holinski B, Everaert D, Mushahwar V, Stein R. Real-time control of walking using recordings from dorsal root ganglia. J Neural Eng. 2013;10(5):056008.
Google Scholar
Mushahwar V, Aoyagi Y, Stein R, Prochazka A. Movements generated by intraspinal microstimulation in the intermediate gray matter of the anesthetized, decerebrate, and spinal cat. Can J Physiol Pharmacol. 2004;82(8–9):702–14.
Google Scholar