- Completion of divestment of first major tranche in Bajaj Joint Ventures | Allianz Allianz.com
- Bajaj Finserv Increases Stake in Insurance Subsidiaries, Joint Venture Ends InvestyWise |
- Bajaj Finserv shares in focus as firm completes acquisition of 23% stake in insurance companies from Allianz SE Upstox
- Allianz Sells Indian Holdings for 2.1 Billion Euros MarketScreener
- Bajaj Finserv Ends Insurance JV With Allianz Group In Rs 21,400-Crore Deal NDTV Profit
Category: 3. Business
-
Completion of divestment of first major tranche in Bajaj Joint Ventures | Allianz – Allianz.com
-

Vanda Pharmaceuticals Announces Receipt of FDA Decision Letter on HETLIOZ® Supplemental New Drug Application for Jet Lag Disorder
WASHINGTON, Jan. 8, 2026 /PRNewswire/ — Vanda Pharmaceuticals Inc. (Vanda) (Nasdaq: VNDA) today announced that it has received a decision letter from the U.S. Food and Drug Administration’s (FDA) Center for Drug Evaluation and Research (CDER) concluding that the supplemental New Drug Application (sNDA) for HETLIOZ® (tasimelteon) for the treatment of jet lag disorder cannot be approved in its current form. This letter stems from CDER’s agreed re-review of the jet lag application under the October 1 collaborative framework agreement.
The FDA acknowledged positive efficacy from Vanda’s controlled clinical trials, however, the FDA concluded that these data do not provide substantial evidence of effectiveness for jet lag disorder, primarily on the grounds that controlled phase advance protocols (5-hour and 8-hour bedtime shifts) are not sufficiently analogous to actual jet travel, which according to the FDA involves additional factors such as reduced oxygen pressure, physical constraints, noise, and lighting changes.
Vanda respectfully disagrees with this interpretation. Phase advance models are widely accepted in circadian rhythm research as valid and reliable surrogates for simulating the core circadian misalignment underlying eastward jet lag—the primary driver of the disorder’s hallmark symptoms per ICSD-3 criteria. These models reproducibly induce the essential features of jet lag without the confounders of variable travel conditions which are unrelated to jet lag. The convergent evidence from Vanda’s studies including simulated and actual transatlantic travel demonstrates tasimelteon’s meaningful benefits on sleep duration, latency to persistent sleep, and next-day alertness.
Tasimelteon’s safety profile is also well-established, with predominantly mild adverse events and a market experience of over 10 years in chronic approved indications. Vanda maintains that the submitted dataset meets the statutory standard for substantial evidence of effectiveness on clinically relevant endpoints, for jet lag disorder.
Procedural Status
As previously announced, in August 2025 the D.C. Circuit set aside a prior FDA refusal to approve HETLIOZ® for jet lag disorder, describing Vanda’s evidence as “specific, reasoned, and rooted in evidence” and the FDA’s prior review as “cursory,” while noting statistically significant improvements on primary endpoints across trials.
Following that ruling, Vanda and the FDA entered a collaborative framework agreement in October 2025, under which the FDA committed to an expedited re-review of the sNDA by January 7, 2026, including consideration of narrowed, sleep-focused indications.
Vanda appreciates the FDA’s engagement but believes the current decision does not fully reflect the collaborative spirit or address the Court’s concerns regarding meaningful engagement with the evidence. Vanda remains committed to working constructively with the FDA while pursuing all appropriate avenues to advance approval of HETLIOZ® for jet lag disorder and make this important therapy available to travelers.
About Vanda Pharmaceuticals Inc.
Vanda is a leading global biopharmaceutical company focused on the development and commercialization of innovative therapies to address high unmet medical needs and improve the lives of patients. For more on Vanda Pharmaceuticals Inc., please visit www.vandapharma.com and follow us on X @vandapharma.
About HETLIOZ®
HETLIOZ® is a melatonin‑receptor agonist, approved in the United States for the treatment of Non‑24‑Hour Sleep‑Wake Disorder and nighttime sleep disturbances associated with Smith‑Magenis Syndrome. For full U.S. Prescribing Information for HETLIOZ®, including indications and Important Safety Information, visit www.hetlioz.com.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
Various statements in this press release, including, but not limited to statements regarding Vanda’s commitment to working with the FDA while pursuing appropriate avenues to advance approval of HETLIOZ® in jet lag disorder, and the potential commercial availability of HETLIOZ® for the treatment of jet lag disorder are “forward-looking statements” under the securities laws. All statements other than statements of historical fact are statements that could be deemed forward-looking statements. Forward-looking statements are based upon current expectations and assumptions that involve risks, changes in circumstances and uncertainties. Important factors that could cause actual results to differ materially from those reflected in Vanda’s forward-looking statements include, among others, the FDA’s willingness to work with Vanda and meaningfully engage with the evidence, the results of Vanda’s efforts to advance and obtain FDA approval of HETLIOZ® in jet lag disorder, and Vanda’s ability to successfully execute a commercial launch of HETLIOZ® for the treatment of jet lag disorder if approved. Therefore, no assurance can be given that the results or developments anticipated by Vanda will be realized, or even if substantially realized, that they will have the expected consequences to, or effects on, Vanda. Forward-looking statements in this press release should be evaluated together with the various risks and uncertainties that affect Vanda’s business and market, particularly those identified in the “Cautionary Note Regarding Forward-Looking Statements”, “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” sections of Vanda’s most recent Annual Report on Form 10-K, as updated by Vanda’s subsequent Quarterly Reports on Form 10-Q, Current Reports on Form 8-K and other filings with the U.S. Securities and Exchange Commission, which are available at www.sec.gov.
All written and verbal forward-looking statements attributable to Vanda or any person acting on its behalf are expressly qualified in their entirety by the cautionary statements contained or referred to herein. Vanda cautions investors not to rely too heavily on the forward-looking statements Vanda makes or that are made on its behalf. The information in this press release is provided only as of the date of this press release, and Vanda undertakes no obligation, and specifically declines any obligation, to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
Corporate Contact:
Kevin Moran
Senior Vice President, Chief Financial Officer and Treasurer
Vanda Pharmaceuticals Inc.
202-734-3400
[email protected]Jim Golden / Jack Kelleher / Dan Moore
Collected Strategies
[email protected]SOURCE Vanda Pharmaceuticals Inc.
Continue Reading
-
North Solihull looks forward to the next Solihull Apprenticeship and Pathways Show
Apprenticeships and post-16 pathways such as BTEC and T Levels will be on display at the show later this month for young people to learn about local opportunities.
With over 40 employers and training providers signed up for the show, this event enables young people to learn about alternative routes after GCSE’s. They can speak to exhibitors and current apprentices who will be able to share their first-hand experience. The first hour is a quiet hour for young people with additional needs to find out more about Inclusive programmes such as Supported Internships.
Visitors will have the chance to speak to employers and training providers from hospitality, manufacturing, construction, engineering and public sectors. Exhibitors include JLR, National Gas, Severn Trent, the NEC Group, Everyone Active, Solihull College & University Centre, Reflections Training Academy, Creative Alliance and Solihull Council. It will take place at the North Solihull Sports Centre on Wednesday 28 January 2026 from 2:30pm to 7:00pm.
Councillor Heather Delaney, Portfolio Holder for Economy, Business & Skills, said: “The Solihull Apprenticeship and Pathways Show has always been well attended. We are excited for new exhibitors joining us this time such as National Gas and British Academy of Jewellery. We are always working to expand our network and reach so that visitors can learn about opportunities from more industries. Speaking directly with employers and current apprentices is the best way for young people to find out if a training programme or apprenticeship is for them.
“At the beginning of the year, it’s usually the time that young people start to think about what they’ll do after GCSE’s. It can be daunting for some, so we hope that an event like this would give young people the chance to get all the information they need to explore all the post-16 pathways available locally. Ultimately, it will help them take the best next steps. I’d encourage all young people, especially those in Year 10-13, to sign up for the show.”
Organised by Solihull Careers Hub which is part of the Council’s Employment and Skills Team, the Solihull Apprenticeship and Pathways Show is free to attend and it is advised to book in advance. Free parking is available on site. For more information or sign up, please visit this webpage.
Editor notes:
- The Solihull Apprenticeship and Pathways Show is funded by the UK Government through the UK Shared Prosperity Fund, West Midlands Combined Authority, The Careers and Enterprise Company and Solihull Metropolitan Borough Council.
- Solihull Careers Hub is funded by The Careers and Enterprise Company and Solihull Metropolitan Borough Council.
Continue Reading
-
Minister Calleary signs new contract with An Post for cash payments at post offices
- An Post will deliver approx. 25 million payments to welfare customers in 2026
- Pensions, jobseekers, child benefit and other payments can be collected at your local post office
Today, Minister for Social Protection, Dara Calleary announced that An Post has been awarded the contract for the Department of Social Protection’s new Cash Payment Services contract.
The Department’s Cash Payment Services contract provides for over-the-counter personal cash payments to welfare recipients. About 30% of payments made to the Department’s customers are made by cash.
The awarding of the contract to An Post follows a competitive tender process to secure the best-value and best-quality provision for customers and taxpayers.
The new Cash Payment Services contract began on the 1st of January 2026 and will expire in December 2028. After the initial three-year contract period is complete, the Department has the option to extend the contract for one additional year.
Welcoming the new contract, Minister Calleary said:
“An Post delivered almost 25 million welfare payments to our customers through the An Post Network in 2025. Now that this new contract is in place, my Department, in conjunction with An Post will continue to provide the important provision of cash payments to the Department’s customers who rely on this service across urban and rural communities.”
Debbie Byrne, Managing Director of An Post Retail said:
“An Post is pleased to continue delivering cash payment services to Department of Social Protection customers across Ireland.”
“Through investment in our unique network of 880 Post Offices, technology, training and our dedicated staff and Postmasters, we will continue to ensure customers’ access to secure, reliable cash payments, so essential to local communities.
Post Offices handled more than €7 billion in DSP payments last year, showing huge resilience during extreme weather events and other challenges to serve customers. We look forward to our continued close working relationship with the Department over the coming years”, she added.
Continue Reading
-
REMINDER: Swisscom 3G phase-out | CERN
Swisscom will switch off its 3G network at the end of 2025. This 3rd generation of mobile communications was introduced 20 years ago and has become inefficient in terms of energy requirements, spectrum usage and the resources required to maintain it, so operators are freeing up these resources for new technologies.
To continue using your mobile phone at CERN (in Switzerland and in CERN’s underground facilities) you’ll need a device that supports at least 4G and VoLTE (Voice over LTE). SMS (Short Message Service) services will be maintained via 4G.
You can check your device’s compatibility via Swisscom’s cockpit or by sending a free SMS to 444, using your CERN SIM card, with the keyword 3G. Please be aware that some models’ characteristics are not properly identified in Swisscom’s database. If this is the case, you should check that 4G/5G is shown on your phone as an option for data connectivity. You will also need a VoLTE-compatible phone to place/receive calls. If your device displays 4G but it disappears when you make a call (while connected to Swisscom), then VoLTE is not switched on or your device is not compatible.
If your phone is not compatible, you should replace it. 4G and VoLTE have been standard on mobile phones for the last 7 to 10 years, depending on the brand.
For further information, you can visit this link or contact the mobile service support team.
__________
This announcement was originally published in June 2025.
Continue Reading
-
Chinese shares close lower Thursday-Xinhua
BEIJING, Jan. 8 (Xinhua) — Chinese stocks closed lower on Thursday, with the benchmark Shanghai Composite Index down 0.07 percent to 4,082.98 points.
The Shenzhen Component Index closed 0.51 percent lower at 13,959.48 points.
The combined turnover of these two indices totaled 2.82 trillion yuan (about 402 billion U.S. dollars), down from 2.85 trillion yuan on the previous trading day.
Stocks related to the commercial aerospace sector as well as brain-computer interfaces and AI applications led the gains, while those related to insurance, securities companies, lithium mining and precious metals experienced declines.
The ChiNext Index, tracking China’s Nasdaq-style board of growth enterprises, fell 0.82 percent to close at 3,302.31 points.
However, the STAR Composite Index, which reflects the performance of stocks on China’s sci-tech innovation board, closed 1.14 percent higher at 1,766.57 points. ■
Continue Reading
-

CR Tested These Protein Powders. All Had Low Levels of Lead.
Before publication, CR contacted the manufacturers of all the products we tested and shared our results and methodology with them. We wanted to know whether they were using any unique sourcing or manufacturing processes that could explain their comparatively cleaner results, and what that might reveal about other manufacturers’ practices.
Premier Protein declined to comment. Representatives from Equate’s parent company, Walmart, and Clean Simple Eats didn’t respond to multiple requests for comment.
Truvani’s co-founder and co-CEO, Derek Halpern, said in an interview that what sets his company apart is the frequency with which it tests for heavy metals. “I’ve been told routinely by my manufacturers that the volume of tests that we ask for far outstrips anyone else they’ve ever worked with,” he said. “I just want a test result for every lot—that doesn’t seem that ridiculous to me.”
Truvani has tested its chocolate-flavored protein powder 162 times over the last 12 months, Halpern said. Every lot of Truvani products is tested for heavy metals and other contaminants, and ingredients that don’t meet internal standards are rejected. (Halpern declined to share the specific thresholds Truvani uses, but said that its lead standard is similar to the California Prop 65 limit that CR uses in its level of concern calculations.)
Halpern said he suspects less rigorous approaches are more common across the industry because they’re less costly and still technically meet FDA requirements. Some companies rely on spot-checks or certificates of analysis from ingredient suppliers instead of testing every finished lot, he said.
“It can be more expensive to ensure that every vat of your product is very low in lead,” says Cohen of Harvard Medical School. “And without a requirement that it be that way, it’s unlikely that the industry as a whole is going to move in that direction.”
Lindsay Dahl, chief impact officer at the supplement brand Ritual, says she thinks that “heavy metal testing transparency is feasible for the entire industry.” Ritual tests its ingredients and all finished goods for contamination, and uses California’s Prop 65 limit as a goalpost for most products, she says.
Ritual is unique in that it publishes detailed sourcing information for its products. “We openly share the final place of manufacturing and the names of our suppliers for the public to see,” says Dahl, who added that the company thinks that “ingredient traceability is the best way to help reduce contaminants.” She noted that the powder tested by CR was made with Puris-brand pea protein from North America and cocoa powder from several countries through Cocoa Horizons, a program that promotes sustainable and traceable farming.
“It took us three years of searching and testing different cocoa suppliers to finally launch a chocolate flavor version of Essential Protein,” says Dahl, who attributed the delay to Ritual’s heavy metal and human rights standards. “While we spend a tremendous amount of time working to find the highest quality suppliers, we also know it’s hard to have formulas that are entirely contaminant-free, which is where our product testing comes in.”
In a letter to Congress last year, Ritual’s CEO, Katerina Schneider, said that because plant-based protein powder is a “high-risk product,” the company publishes heavy metal test results for one recently released lot of each flavor of its Essential Protein powder on its website. In the letter, Schneider also took the rare step of advocating for greater industry regulation, calling on Congress to “empower the FDA to establish health-protective limits for heavy metals in supplements and protein powder.”
It’s a position also held by CR’s consumer advocates—and many others. A CR petition calling on the FDA to set strict standards for heavy metals in protein supplements has garnered over 43,000 signatures since October.
“The FDA is still lacking enforceable lead limits for protein powders and dietary supplements,” says Brian Ronholm, CR’s director of food policy. “Having these standards in place would push the industry to consistently make products with lower levels of lead, which our test results certainly demonstrate is possible for companies to do.”
Continue Reading
-

A year of action: More than 43,000 counterfeit products removed from Manchester’s streets in 2025
In 2025 Manchester City Council’s Trading Standards Team seized and destroyed nearly £4.5m of counterfeit goods.
Ranging from fake handbags, trainers, jewellery, electronic items, sportswear, to children’s toys and sunglasses there are few areas that the counterfeit goods industry does not reach.
However, through exemplary partnership work alongside Greater Manchester Police, and brand representatives this criminal industry has taken a substantial hit over the past 12 months.
Of the more than 43,500 counterfeit items which were seized it is estimated that the value lost to the industry was between £34m – £43m.*
In addition to counterfeit goods a substantial push was made throughout the year to crack down on the sale and distribution of illicit tobacco. Sold in packaging not compliant with UK law and often shipped in from oversees, it presents a substantial impediment to supporting Mancunians to quit smoking and move away from tobacco products.
As Manchester has some of the worst health outcomes in the country when it comes to smoking-related illnesses it is hugely important that steps are taken to curtail the sale of illicit tobacco.
In total, 316,625 cigarettes – equivalent to nearly 16,000 individual packs were seized. In addition, 258kg of hand rolling tobacco was seized, as well as more than 18,000 illegal vapes which do not comply with UK laws or regulations.
Councillor Lee-Ann Igbon, Executive Member for Vibrant Neighbourhoods, said: “I am incredibly proud of the results that our officers achieved throughout 2025. The counterfeit industry was substantially embedded in our communities, but through their diligence and the support of our valued partners we have driven away some of the worst offenders and are beginning the process of regenerating the areas of Manchester that were long blighted by this sort of crime.
“Through Operations Elswick and Machinize run in collaboration with GMP we have made a significant impact against criminal enterprises and we hope this sends a message that we will not tolerate this harmful trade.”
Detective Chief Inspector Melanie Johnson, lead coordinator over Operation Machinize for GMP, said: “Last year we collaborated with Manchester City Council’s Trading Standards to tackle businesses on our high streets that were being used as a front for criminality and putting our communities at risk.
“As a result of our operations, we managed to seize over £1 million worth of illegal items.
“The joint partnership operation has enabled GMP to gather further information and intelligence enhancing our understanding of criminality within these types of businesses.
“We take any information we receive very seriously and will continue to investigate all aspects of this criminality to protect our communities from the harms of illegal products.”
*Note on Lost Value
This is the estimated loss of money when comparing the price of a sold counterfeit item, vs the authentic product. Ie., if a pair of counterfeit Nike shoes were sold for £20, when the RRP was £90, the lost value would be £70.
Continue Reading
-

DMA Review contributions
Today, the European Commission published a summary and the individual contributions received in response to the consultation on the ongoing review of the Digital Markets Act (DMA).
The Commission welcomes the high level of participation, with over 450 contributions submitted by a broad range of interested parties, including small and medium-sized enterprises (SMEs), gatekeepers, civil society organisations, academics, and individual citizens. The contributions generally show respondents’ broad support for the DMA’s objectives and indicate that the regulation has already brought benefits. Some contributions ask to strengthen interoperability, data access and data portability, as well as support for SMEs. Some also ask to expand the DMA’s scope, particularly in relation to AI and cloud services. Gatekeepers on the other hand expressed criticisms such as regarding impact on user experience, as well as concerns about proportionality.
The assessment of these contributions will feed into the Commission’s review report to be presented by 3 May 2026 to the European Parliament, the Council, and the European Economic and Social Committee. The regular review of the DMA every three years is a legal requirement, mandated by the regulation itself, to ensure that the DMA meets its objectives and maintains its effectiveness in the evolving landscape of digital markets.
The public consultation, which was launched on 3 July 2025 as part of the ongoing review, was accompanied by a call for evidence and a dedicated questionnaire on Artificial Intelligence (AI), which were published on 26 August 2025. The contributions to the call for evidence are already public.
See the announcement also on Commission’s press corner.
Continue Reading
-
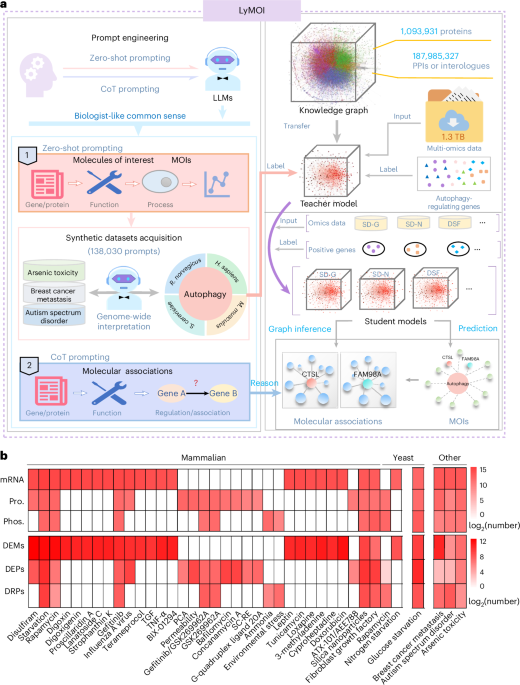
A deep learning and large language hybrid workflow for omics interpretation
Stefely, J. A. et al. Mitochondrial protein functions elucidated by multi-omic mass spectrometry profiling. Nat. Biotechnol. 34, 1191–1197 (2016).
Google Scholar
Karczewski, K. J. & Snyder, M. P. Integrative omics for health and disease. Nat. Rev. Genet. 19, 299–310 (2018).
Google Scholar
Rhodes, D. R. & Chinnaiyan, A. M. Integrative analysis of the cancer transcriptome. Nat. Genet. 37, S31–S37 (2005).
Google Scholar
Chung, M. et al. Best practices on the differential expression analysis of multi-species RNA-seq. Genome Biol. 22, 121 (2021).
Google Scholar
Yamada, R. et al. Interpretation of omics data analyses. J. Hum. Genet. 66, 93–102 (2021).
Google Scholar
Subramanian, I. et al. Multi-omics data integration, interpretation, and its application. Bioinform. Biol. Insights 14, 1177932219899051 (2020).
Google Scholar
Shu, T. et al. Plasma proteomics identify biomarkers and pathogenesis of COVID-19. Immunity 53, 1108–1122.e5 (2020).
Google Scholar
Shui, K. et al. Small-sample learning reveals propionylation in determining global protein homeostasis. Nat. Commun. 14, 2813 (2023).
Google Scholar
Yuan, Y. et al. PIM1 promotes hepatic conversion by suppressing reprogramming-induced ferroptosis and cell cycle arrest. Nat. Commun. 13, 5237 (2022).
Google Scholar
Hirschberg, J. & Manning, C. D. Advances in natural language processing. Science 349, 261–266 (2015).
Google Scholar
ChatGPT: Optimizing Language Models for Dialogue (OpenAI, 2022).
Christiano, P. F. et al. Deep reinforcement learning from human preferences. In Proc. 31st International Conference on Neural Information Processing Systems (eds von Luxburg, U. et al.) 4302–4310 (Curran, 2017).
Brown, T. B. et al. Language models are few-shot learners. In Proc. 34th Conference on Neural Information Processing Systems (eds Larochelle, H. et al.) 1–25 (2020).
Wei, J. S. et al. Chain-of-thought prompting elicits reasoning in large language models. In Proc. 36th International Conference on Neural Information Processing Systems (eds Koyejo, S. et al.) 24824–24837 (Curran, 2022).
Klionsky, D. J. et al. Guidelines for the use and interpretation of assays for monitoring autophagy (4th edition). Autophagy 17, 1–382 (2021).
Google Scholar
Ulgherait, M. et al. Circadian autophagy drives iTRF-mediated longevity. Nature 598, 353–358 (2021).
Google Scholar
Skrott, Z. et al. Alcohol-abuse drug disulfiram targets cancer via p97 segregase adaptor NPL4. Nature 552, 194–199 (2017).
Google Scholar
Zhao, M.-M. et al. Novel cleavage sites identified in SARS-CoV-2 spike protein reveal mechanism for cathepsin L-facilitated viral infection and treatment strategies. Cancer Discov. 8, 53 (2022).
Google Scholar
Podgorski, J. & Berg, M. Global threat of arsenic in groundwater. Science 368, 845–850 (2020).
Google Scholar
Diamantopoulou, Z. et al. The metastatic spread of breast cancer accelerates during sleep. Nature 607, 156–162 (2022).
Google Scholar
Obradović, M. M. S. et al. Glucocorticoids promote breast cancer metastasis. Nature 567, 540–544 (2019).
Google Scholar
Hirota, T. & King, B. H. Autism spectrum disorder: a review. JAMA 329, 157–168 (2023).
Google Scholar
Chen, A. et al. Spatiotemporal transcriptomic atlas of mouse organogenesis using DNA nanoball-patterned arrays. Cell 185, 1777–1792.e21 (2022).
Google Scholar
Tang, F. et al. A pan-cancer single-cell panorama of human natural killer cells. Cell 186, 4235–4251.e20 (2023).
Google Scholar
Velmeshev, D. et al. Single-cell analysis of prenatal and postnatal human cortical development. Science 382, eadf0834 (2023).
Google Scholar
Deng, W. et al. THANATOS: an integrative data resource of proteins and post-translational modifications in the regulation of autophagy. Autophagy 14, 296–310 (2018).
Google Scholar
Han, Z. et al. Model-based analysis uncovers mutations altering autophagy selectivity in human cancer. Nat. Commun. 12, 3258 (2021).
Google Scholar
Santos, A. et al. A knowledge graph to interpret clinical proteomics data. Nat. Biotechnol. 40, 692–702 (2022).
Google Scholar
Zhang, Z. et al. Large graph models: a perspective. Preprint at https://doi.org/10.48550/arXiv.2308.14522 (2023).
Yu, H. et al. Annotation transfer between genomes: protein–protein interologs and protein–DNA regulogs. Genome Res. 14, 1107–1118 (2004).
Google Scholar
Lyu, Y., Huang, X. & Zhang, Z. Revisiting 2D convolutional neural networks for graph-based applications. IEEE Trans. Pattern Anal. Mach. Intell. 45, 6909–6922 (2023).
Google Scholar
Díez, J., Walter, D., Muñoz-Pinedo, C. & Gabaldón, T. DeathBase: a database on structure, evolution and function of proteins involved in apoptosis and other forms of cell death. Cell Death Differ. 17, 735–736 (2010).
Google Scholar
Homma, K., Suzuki, K. & Sugawara, H. The Autophagy Database: an all-inclusive information resource on autophagy that provides nourishment for research. Nucleic Acids Res. 39, D986–D990 (2011).
Google Scholar
Moussay, E. et al. The acquisition of resistance to TNFα in breast cancer cells is associated with constitutive activation of autophagy as revealed by a transcriptome analysis using a custom microarray. Autophagy 7, 760–770 (2011).
Google Scholar
Xu, J. & Li, Y. H. miRDeathDB: a database bridging microRNAs and the programmed cell death. Cell Death Differ. 19, 1571 (2012).
Google Scholar
Arntzen, M., Bull, V. H. & Thiede, B. Cell death proteomics database: consolidating proteomics data on cell death. J. Proteome Res. 12, 2206–2213 (2013).
Google Scholar
Wanichthanarak, K., Cvijovic, M., Molt, A. & Petranovic, D. yApoptosis: yeast apoptosis database. Database 2013, bat068 (2013).
Google Scholar
Türei, D. et al. Autophagy Regulatory Network—a systems-level bioinformatics resource for studying the mechanism and regulation of autophagy. Autophagy 11, 155–165 (2015).
Google Scholar
Wu, D. et al. ncRDeathDB: a comprehensive bioinformatics resource for deciphering network organization of the ncRNA-mediated cell death system. Autophagy 11, 1917–1926 (2015).
Google Scholar
Wang, N. N. et al. HAMdb: a database of human autophagy modulators with specific pathway and disease information. J. Cheminform. 10, 34 (2018).
Google Scholar
Chen, K. et al. Autophagy and Tumor Database: ATdb, a novel database connecting autophagy and tumor. Database https://doi.org/10.1093/database/baaa052 (2020).
Zhou, N. & Bao, J. FerrDb: a manually curated resource for regulators and markers of ferroptosis and ferroptosis–disease associations. Database https://doi.org/10.1093/database/baaa021 (2020).
Zhang, L. et al. MCDB: a comprehensive curated mitotic catastrophe database for retrieval, protein sequence alignment, and target prediction. Acta Pharm. Sin. B 11, 3092–3104 (2021).
Google Scholar
Sun, Y. J., Sheng, D. F., Zhou, Z. H. & Wu, Y. F. AI hallucination: towards a comprehensive classification of distorted information in artificial intelligence-generated content. Hum. Soc. Sci. Commun. https://doi.org/10.1057/S41599-024-03811-X (2024).
Bang, Y. et al. A multitask, multilingual, multimodal evaluation of ChatGPT on reasoning, hallucination, and interactivity. Preprint at https://arxiv.org/abs/2302.04023 (2023).
Zhu, W., Swaminathan, G. & Plowey, E. D. GA binding protein augments autophagy via transcriptional activation of BECN1-PIK3C3 complex genes. Autophagy 10, 1622–1636 (2014).
Google Scholar
Sun, W., Jia, M., Feng, Y. & Cheng, X. Lactate is a bridge linking glycolysis and autophagy through lactylation. Autophagy 19, 3240–3241 (2023).
Google Scholar
Fujioka, Y. et al. Structural basis of starvation-induced assembly of the autophagy initiation complex. Nat. Struct. Mol. Biol. 21, 513–521 (2014).
Google Scholar
Schreiber, A. et al. Multilayered regulation of autophagy by the Atg1 kinase orchestrates spatial and temporal control of autophagosome formation. Mol. Cell 81, 5066–5081.e10 (2021).
Google Scholar
Feng, Y. et al. Phosphorylation of Atg9 regulates movement to the phagophore assembly site and the rate of autophagosome formation. Autophagy 12, 648–658 (2016).
Google Scholar
Cowley, M. J. et al. PINA v2.0: mining interactome modules. Nucleic Acids Res. 40, D862–D865 (2012).
Google Scholar
Das, J. & Yu, H. HINT: high-quality protein interactomes and their applications in understanding human disease. BMC Syst. Biol. 6, 92 (2012).
Google Scholar
Razick, S., Magklaras, G. & Donaldson, I. M. iRefIndex: a consolidated protein interaction database with provenance. BMC Bioinformatics 9, 405 (2008).
Google Scholar
Oughtred, R. et al. The BioGRID interaction database: 2019 update. Nucleic Acids Res. 47, D529–D541 (2019).
Google Scholar
Calderone, A., Castagnoli, L. & Cesareni, G. mentha: a resource for browsing integrated protein-interaction networks. Nat. Methods 10, 690–691 (2013).
Google Scholar
Kotlyar, M., Pastrello, C., Malik, Z. & Jurisica, I. IID 2018 update: context-specific physical protein–protein interactions in human, model organisms and domesticated species. Nucleic Acids Res. 47, D581–D589 (2019).
Google Scholar
Li, T. et al. A scored human protein–protein interaction network to catalyze genomic interpretation. Nat. Methods 14, 61–64 (2017).
Google Scholar
Galluzzi, L. et al. Molecular definitions of autophagy and related processes. EMBO J. 36, 1811–1836 (2017).
Google Scholar
Yi, C. et al. Formation of a Snf1-Mec1-Atg1 module on mitochondria governs energy deprivation-induced autophagy by regulating mitochondrial respiration. Dev. Cell 41, 59–71.e54 (2017).
Google Scholar
Yi, C., Tong, J. J. & Yu, L. Mitochondria: the hub of energy deprivation-induced autophagy. Autophagy 14, 1084–1085 (2018).
Google Scholar
Clement, S. T., Dixit, G. & Dohlman, H. G. Regulation of yeast G protein signaling by the kinases that activate the AMPK homolog Snf1. Sci. Signal. 6, ra78 (2013).
Google Scholar
Mok, J. et al. Deciphering protein kinase specificity through large-scale analysis of yeast phosphorylation site motifs. Sci. Signal. 3, ra12 (2010).
Google Scholar
Asano, S. et al. Direct phosphorylation and activation of a Nim1-related kinase Gin4 by Elm1 in budding yeast. J. Biol. Chem. 281, 27090–27098 (2006).
Google Scholar
Hu, Y. et al. The disulfiram/copper complex induces autophagic cell death in colorectal cancer by targeting ULK1. Front. Pharmacol. 12, 752825 (2021).
Google Scholar
Jivan, R. et al. Disulfiram with or without metformin inhibits oesophageal squamous cell carcinoma in vivo. Cancer Lett. 417, 1–10 (2018).
Google Scholar
Wu, X. et al. Suppressing autophagy enhances disulfiram/copper-induced apoptosis in non-small cell lung cancer. Eur. J. Pharmacol. 827, 1–12 (2018).
Google Scholar
Xu, S. et al. Inhibition of cathepsin L alleviates the microglia-mediated neuroinflammatory responses through caspase-8 and NF-κB pathways. Neurobiol. Aging 62, 159–167 (2018).
Google Scholar
Liu, H. et al. Oxidized DJ-1 activates the p-IKK/NF-κB/Beclin1 pathway by binding PTEN to induce autophagy and exacerbate myocardial ischemia-reperfusion injury. Eur. J. Pharmacol. 971, 176496 (2024).
Google Scholar
Tate, J. G. et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 47, D941–D947 (2019).
Google Scholar
Kenig, S., Frangež, R., Pucer, A. & Lah, T. Inhibition of cathepsin L lowers the apoptotic threshold of glioblastoma cells by up-regulating p53 and transcription of caspases 3 and 7. Apoptosis 16, 671–682 (2011).
Google Scholar
Zhao, M. M. et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. https://doi.org/10.1038/s41392-021-00558-8 (2021).
Sudhan, D. R., Pampo, C., Rice, L. & Siemann, D. W. Cathepsin L inactivation leads to multimodal inhibition of prostate cancer cell dissemination in a preclinical bone metastasis model. Int. J. Cancer 138, 2665–2677 (2016).
Google Scholar
Richard, V. et al. The double agents in liquid biopsy: promoter and informant biomarkers of early metastases in breast cancer. Mol. Cancer 21, 95 (2022).
Google Scholar
Xu, J. et al. ATP11B inhibits breast cancer metastasis in a mouse model by suppressing externalization of nonapoptotic phosphatidylserine. J. Clin. Invest. https://doi.org/10.1172/jci149473 (2022).
Jiang, C. C. et al. Signalling pathways in autism spectrum disorder: mechanisms and therapeutic implications. Signal Transduct. Target. Ther. 7, 229 (2022).
Google Scholar
Bheda, A., Creek, K. E. & Pirisi, L. Loss of p53 induces epidermal growth factor receptor promoter activity in normal human keratinocytes. Oncogene 27, 4315–4323 (2008).
Google Scholar
Linder, M. et al. EGFR is required for FOS-dependent bone tumor development via RSK2/CREB signaling. EMBO Mol. Med. https://doi.org/10.15252/emmm.201809408 (2018).
Rives, A. et al. Biological structure and function emerge from scaling unsupervised learning to 250 million protein sequences. Proc. Natl Acad. Sci. USA 118, e2016239118 (2021).
Google Scholar
Madani, A. et al. Large language models generate functional protein sequences across diverse families. Nat. Biotechnol. 41, 1099–1106 (2023).
Google Scholar
Yang, F. et al. scBERT as a large-scale pretrained deep language model for cell type annotation of single-cell RNA-seq data. Nat. Mach. Intell. 4, 852 (2022).
Google Scholar
Theodoris, C. V. et al. Transfer learning enables predictions in network biology. Nature 618, 616–624 (2023).
Google Scholar
Cui, H. T. et al. scGPT: toward building a foundation model for single-cell multi-omics using generative AI. Nat. Methods 21, 1470–1480 (2024).
Google Scholar
Wu, A. et al. Causality for large language models. Preprint at https://arxiv.org/abs/2410.15319 (2024).
Lee, S. et al. Reasoning abilities of large language models: in-depth analysis on the abstraction and reasoning corpus. Preprint at https://arxiv.org/abs/2403.11793 (2024).
Kipf, T. N. & Welling, M. Variational graph auto-encoders. Preprint at https://arxiv.org/abs/1611.07308 (2016).
Wu, Z. H. et al. A comprehensive survey on graph neural networks. IEEE Trans. Neural Netw. Learn. Syst. 32, 4–24 (2021).
Google Scholar
Miao, Z., Humphreys, B. D., McMahon, A. P. & Kim, J. Multi-omics integration in the age of million single-cell data. Nat. Rev. Nephrol. 17, 710–724 (2021).
Google Scholar
Ma, A. et al. Integrative methods and practical challenges for single-cell multi-omics. Trends Biotechnol. 38, 1007–1022 (2020).
Google Scholar
Zhang, Y. et al. DeepPhagy: a deep learning framework for quantitatively measuring autophagy activity in Saccharomyces cerevisiae. Autophagy 16, 626–640 (2020).
Google Scholar
Tang, D., Zhang, C., Peng, D. & Xue, Y. Transcriptome of Saccharomyces cerevisiae during glucose starvation. Datasets. SRA https://www.ncbi.nlm.nih.gov/sra/?term=PRJNA912308 (2025).
Tang, D., Zhang, C., Peng, D. & Xue, Y. The proteome and phosphoproteome of Saccharomyces cerevisiae during glucose starvation. Datasets. iProX https://www.iprox.cn//page/project.html?id=IPX0005607000 (2025).
Tang, D., Zhang, C., Peng, D. & Xue, Y. LyMOI: large hybrid models for omics interpretation. Source code. GitHub https://github.com/BioCUCKOO/LyMOI (2025).
Continue Reading