Farmers across Brazil have begun planting the 2025/26 crop season, with expectations for another record in corn and soybean acreage. The first outlook for the new cycle, released by the National Supply Company (Conab), Brazil’s food supply and statistics agency, projects an increase in planted area. The expansion is driven by growing domestic demand for biofuels and strong export performance, which continues to push shipments to record levels. Despite the expected acreage growth, gross margins for both crops are likely to decline significantly due to rising production costs and lower prices. This article reviews the first official Brazilian government estimates for soybeans and corn, analyzes financial trends, and explores their implications for the global soybean and corn trade.
Soybean Acreage Expected to Hit Historical Highs
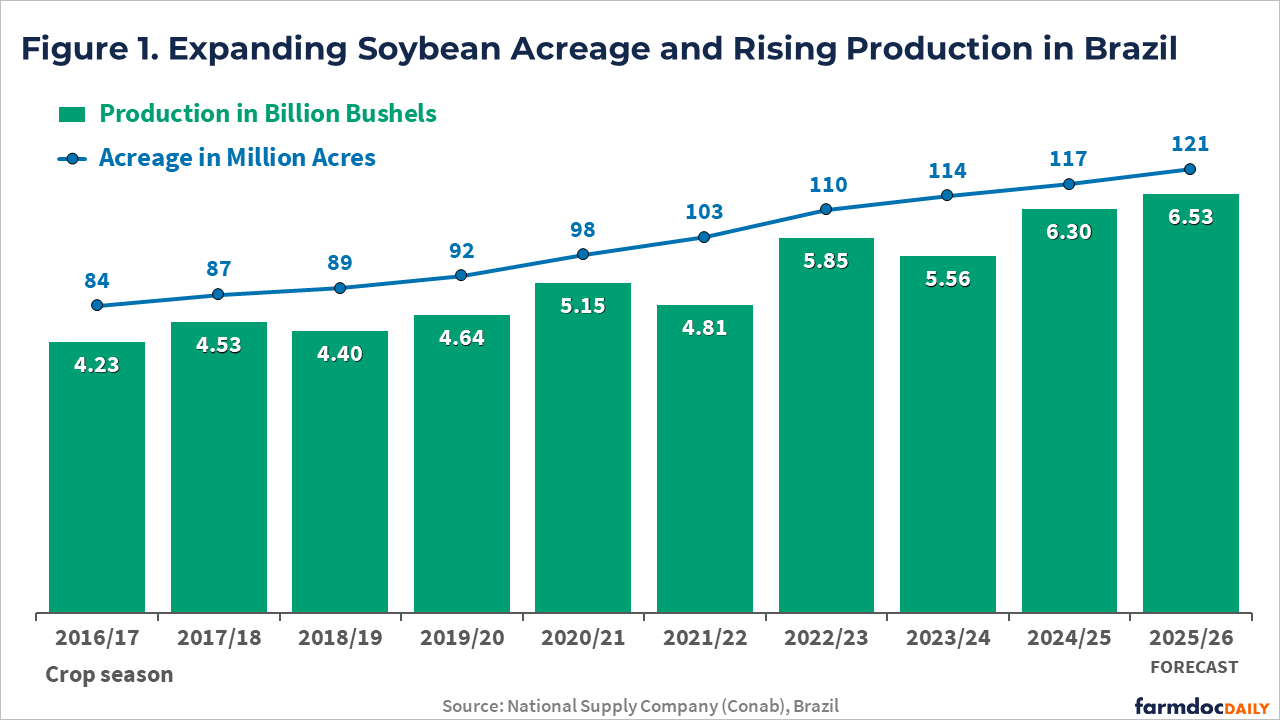
In its preliminary estimate released on October 14, Conab projected that Brazil’s soybean acreage will increase by 3.5%, reaching 121 million acres – the largest area on record. For comparison, U.S. farmers planted 81 million acres of soybeans in the current crop season. If weather conditions cooperate, Brazilian production is expected to reach a new record of 6.5 billion bushels, up 3.6% from last season (see Figure 1). As of October 11, approximately 11% of the soybean area had been planted, below the five-year average of 17%, according to Conab.
The expansion in soybean acreage is being driven by strong domestic and international demand. On the domestic side, growth is supported by Brazil’s biodiesel mandate, with soybean oil being the main raw ingredient. As of August 1, 2025, the country raised its required biodiesel blend from B14 to B15. Basically, diesel fuel must now contain 15% biodiesel, up from 14%, which represents about a 7% increase in total biodiesel use, given Brazil’s large diesel market. The change is part of the government’s Fuel of the Future program, aimed at boosting domestic biofuel production, creating jobs, and reducing dependence on imported fossil fuels.
On the international side, demand remains strong, particularly from China. From January through September this year, Brazil exported 3.45 billion bushels of soybeans, with 77% of that volume shipped to China, according to the Foreign Trade Secretariat (Secex/Brazil). The total is 5% higher than in the same period last year. Brazil is projected to reach a record 3.9 billion bushels in soybean exports by the end of 2025, with shipments expected to surpass 4 billion bushels next year (see Figure 2).

A significant share of Brazil’s export growth to China reflects the gap left by the United States, as Chinese buyers have largely suspended purchases of U.S. soybeans since May following the imposition of U.S. tariffs. Historically, China has been the top buyer of U.S. soybeans by a large margin. In 2024, the United States shipped nearly 985 million bushels to China, accounting for 51% of the nation’s total soybean exports that year (Colussi & Langemeier, 2025). China’s current share of Brazil’s soybean exports is historically high – comparable only to 2018, when President Trump launched the first trade war with China (see farmdoc daily, May 15, 2025).
Despite the optimistic outlook for exports, Brazilian farmers are planting a soybean crop that is expected to be financially challenging, with 4% higher production costs and lower international prices. Projections from the Center for Advanced Studies on Applied Economics (Cepea) at the University of São Paulo (USP) indicate a decline in producers’ gross margins for the upcoming season. Compared with last year, average gross margins (gross revenue minus direct production costs) are estimated to fall from $165 per acre to $105 per acre. However, when interest rates, land rent, and depreciation are considered, margins are projected to turn negative (i.e., -$90 per acre).
Corn Acreage to Expand Despite Lower Output
Like soybeans, Brazilian corn acreage is also expected to expand by 4% in the 2025/26 season, reaching 56 million acres, according to Conab’s initial estimates. For comparison, U.S. farmers planted 99 million acres this season. Brazil’s total corn production is projected at 5.46 billion bushels across the country’s three crop cycles – 2% lower than last year and roughly one-third of total U.S. production (see Figure 3). The projected decline reflects an expected reduction in yields, following last season’s record-high production and productivity.

The first corn crop – planted beginning in September across southern Brazil and some irrigated areas of the Southeast – is expected to expand by 6% compared with last year. The second crop, planted right after the soybean harvest in January and February across the Center-West region and now accounting for nearly 80% of Brazil’s total corn production, is projected to see a 4% increase in acreage from last season. The expansion is driven mainly by rising domestic corn consumption, which Conab projects will grow from 3.58 billion bushels to 3.74 billion bushels – supported by stronger demand from the livestock feed and corn ethanol industries. Historically known for its sugarcane-based ethanol, Brazil has been rapidly expanding corn-based ethanol production, which today represents about 20% of the country’s total biofuel output (see farmdoc daily, April 14, 2025).
In addition to stronger domestic demand, corn production is also expected to benefit from higher export projections for next year. While exports for the 2024/25 season are estimated at 1.58 billion bushels, the Brazilian government forecasts shipments of 1.83 billion bushels for the upcoming season – a 16% increase compared with the previous year, though still below the record set in 2022 when China opened its market to Brazilian corn (see Figure 4).

In terms of production costs, Brazilian corn farmers are expected to see a 6% decrease in the next season, according to data from Cepea/USP. Even so, the projected gross margin (gross revenue minus direct production costs) is expected to fall from $75 per acre in the last crop season to just $6 per acre in the 2025/26 cycle. When other expenses, such as land rent and interest, are included, profits turn into losses, estimated at $135 per acre. The projected reduction is based mainly on the historical average yield of 90 bushels per acre, compared with 109 bushels per acre in last season’s record crop.
Final Considerations
Brazil is beginning its summer crop season with expectations of record planted area for both soybeans and corn, driven primarily by rising domestic demand for biofuels and favorable conditions in international markets. In the case of soybeans, higher exports to China – following the suspension of Chinese purchases of U.S. soybeans – have kept prices firm in Brazil, even after a record harvest. For corn, which also reached record output last season, the expansion in planted area is largely supported by the rapid growth of the corn ethanol industry in the Center-West states, a new and quickly expanding sector in Brazil.
However, shrinking profit margins and higher financial costs could slow the pace of expansion ahead. While Brazil’s farmers continue to gain ground in global markets, their profitability is being squeezed by rising input costs, lower commodity prices, and high interest rates – a scenario similar to that faced by U.S. agriculture (see Langemeier et al., 2025), though with some regional differences. If margins remain tight, Brazilian future production growth should rely more on efficiency gains – such as higher yields and investments in logistics and storage – than on further area expansion.
Data and References
Colussi, J., M. Langemeier. “U.S. Soybean Harvest Starts with No Sign of Chinese Buying as Brazil Sets Export Record.” Center for Commercial Agriculture, Purdue University, September 22, 2025.
Colussi, J., J. Coppess, N. Paulson and G. Schnitkey. “Trade Conflicts and Long-Term Consequences: Are Soybeans Doomed to Repeat History?” farmdoc daily (15):90, Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, May 15, 2025.
Colussi, J., G. Schnitkey and N. Paulson. “Ethanol Boom Drives Sharp Rise in Brazil’s Corn Consumption.” farmdoc daily (15):69, Department of Agricultural and Consumer Economics, University of Illinois at Urbana-Champaign, April 14, 2025.
Conab, National Supply Company. Crops Time Series. Soybean and Corn Production. Brasília, Brasil. October 2025.
Langemeier, M., M. Boehlje, and J. Colussi. “Financial Stress on Crop Farms: Who Is Most at Risk in the 2024–26 Downturn?” Center for Commercial Agriculture, Purdue University, September 3, 2024.
Secex, Brazilian Secretariat of Foreign Trade. Brazil’s Ministry of Development, Industry, and Trade. Exports Report. October 2025. http://comexstat.mdic.gov.br/en/geral