Significant
Category: 6. Health
-

Persistent Chemotherapy-Induced Alopecia, Related Distress More Common in Asian Women With Breast Cancer
racial and ethnic disparities were observed in both the incidence and psychological effects of persistent chemotherapy-inducedalopecia (PCIA) among women withbreast cancer , with Asian women experiencing the greatest burden,… -

Can we prevent AI from acting like a sociopath?
Artificial intelligence boosters predict that AI will transform life on Earth for the better. Yet there’s a major problem — artificial intelligence’s alarming propensity for sociopathic behavior.
Large language models (LLMs) like…
Continue Reading
-
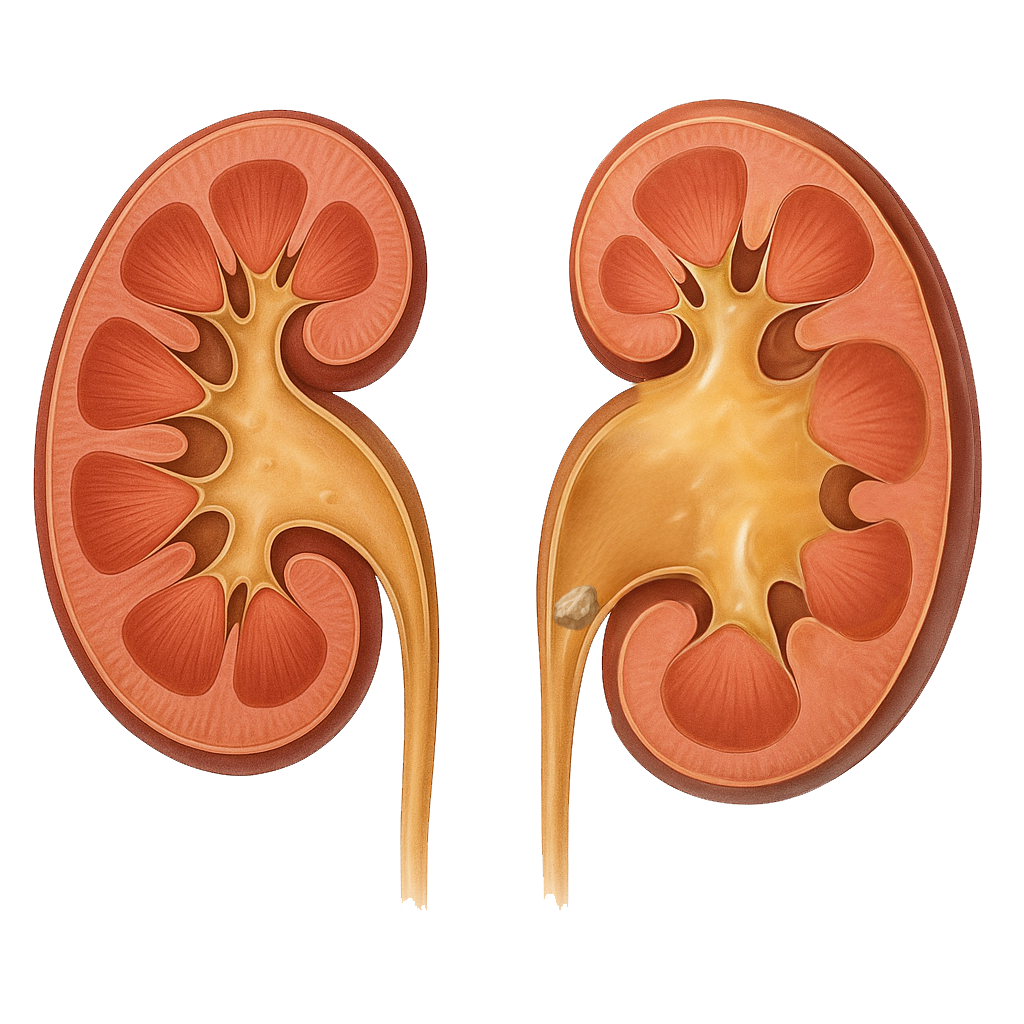
What is hydronephrosis? | Causes of hydronephrosis
NHS. Hydronephrosis [online]. Nhs.uk, England; Jul 2025 [Accessed 2 September 2025]. Available from: Hydronephrosis – NHS
Preminger, G. Urinary Tract Obstruction [online]. MSD Manual, Merck & Co, Inc., Rahway, NJ, USA; Jul…
Continue Reading
-

Faith’s journey from cardiac arrest to recovery inspires a new year of celebration
Hours before ushering in 2025, 18-year-old Faith was helping her father, Troy, prepare for their New Year’s Eve party. Soon, their home would be filled with friends and family. Fresh cookies baked in the oven, filling the house with a…Continue Reading
-
DuPage County Health Department Reports Increase in Respiratory Illnesses – DuPage County Health
- DuPage County Health Department Reports Increase in Respiratory Illnesses DuPage County Health
- Severe flu season overwhelming Chicago area hospitals with more patients visiting ERs CBS News
- Lawrence County residents warned to get vaccinated
Continue Reading
-
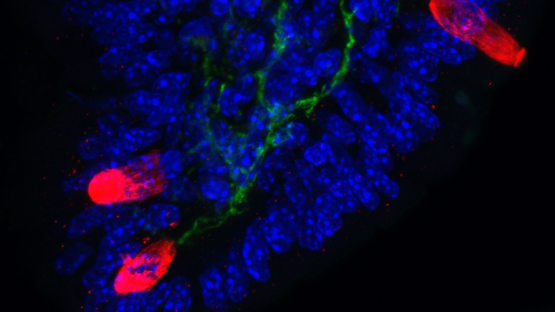
Pain-sensing neurons kick-start immune responses
Pain sensing neurons in the gut kindle inflammatory immune responses that cause allergies and asthma, according to a new study by Weill Cornell Medicine.
The findings, published Jan. 7 in Nature, suggest that current drugs may not be as effective…
Continue Reading
-

Are Food Preservatives Linked to Increased Cancer Risk
Higher intake of food preservatives, widely used in industrially processed foods and beverages to extend shelf life, is associated with a modestly increased risk of cancer, according to the results of a French study published by Hasenböhler…
Continue Reading
-

Face masks ‘inadequate’ and should be swapped for respirators, WHO is advised | Global health
Surgical face masks provide inadequate protection against flu-like illnesses including Covid, and should be replaced by respirator-level masks – worn every time doctors and nurses are face to face with a patient, according to a group of experts…
Continue Reading
-

Application value of QRS wave potential in predicting left ventricular
Introduction
Acute myocardial infarction (AMI) is a critical endpoint of coronary heart disease (CAD), characterized by plaque rupture and acute thrombosis due to coronary atherosclerosis, leading to severe myocardial ischemia and tissue…
Continue Reading
-

Planet-friendly Proteins | World Resources Institute
One of the biggest food trends right now seems to be protein. Everyone from social media influencers to food companies to governments are telling us to eat more of it.
The new U.S. dietary recommendations are the latest to join the fray….
Continue Reading