- Fewer flu cases after spike in Oklahoma amid holiday season Oklahoma Voice
- Oklahoma City Public Schools implements measures to combat rising flu cases KOCO
- Arkansas flu cases ranked ‘high’ by CDC: The latest numbers KHBS
- Health officials warn of…
Category: 6. Health
-
Fewer flu cases after spike in Oklahoma amid holiday season – Oklahoma Voice
-

Fred Hutch experts available to discuss new nutrition guidelines, women’s health, and early cancer detection
Seattle — Jan. 8, 2026 — Fred Hutch Cancer Center researchers have science-informed advice to help people improve their health in the new year. From trending topics to tried-and-true guidance, Fred Hutch is a leader in cancer prevention…
Continue Reading
-

Scientists succeed in synthesising promising anti-cancer fungal compound
Scientists succeed in synthesising promising anti-cancer fungal compound – Daily Times
…Continue Reading
-

Merck Statement on the U.S. Child and Adolescent Immunization Schedule
January 8, 2026 4:57 pm EST
Rahway, N.J., Jan. 8, 2026 – At Merck, we share the same fundamental belief with health care providers and parents…
Continue Reading
-

HHS encourages prevention due to uptick in ND influenza activity – Health and Human Services North Dakota (.gov)
- HHS encourages prevention due to uptick in ND influenza activity Health and Human Services North Dakota (.gov)
- Why flu seems to be everywhere — even if ‘super flu’ is not a thing statnews.com
- ‘Unprecedented’: Western New York hospitals…
Continue Reading
-
Amid conflicting vaccine recommendations, Americans are less likely to trust Trump’s CDC
Americans are now more likely to trust the American Medical Association than the Centers for Disease Control and Prevention when the two conflict on vaccine guidance.
The survey, created by APPC’s Annenberg Health and Risk…
Continue Reading
-
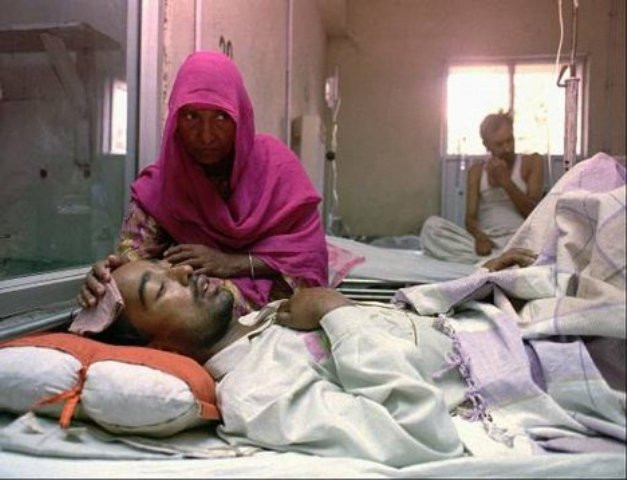
Pindi reels under influenza onslaught
RAWALPINDI:A sharp increase in influenza and respiratory illness cases has…
Continue Reading
-

Are you taking supplements correctly? Here’s a guide on their dosage limits | Well actually
There are more than 100,000 supplements on the US market – capsules, powders, tablets and gummies sold to improve or maintain health. Supplements can contain vitamins, minerals, botanicals and amino acids on their own or in various…
Continue Reading
-

New Yale Study Reveals Mechanism Behind Persistent Autoimmune Joint Destruction
The researchers discovered that these T lymphocytes are expanded in mouse models of joint inflammation and that simply transferring these particular cells to healthy mice caused RA-like joint inflammation. The research team went on to identify…
Continue Reading
-
DOH-Orange Observes Cervical Cancer Awareness Month – Florida Department of Health in Orange County (.gov)
- DOH-Orange Observes Cervical Cancer Awareness Month Florida Department of Health in Orange County (.gov)
- A woman dies from cervical cancer every two minutes, UN says UN News
- Health Dept. Urges Females Age 21+ Get Tested for Cervical Cancer
Continue Reading