Ever slept-in during the weekends without a worry in the world?
Well, new research from US…

Ever slept-in during the weekends without a worry in the world?
Well, new research from US…

Barnsley has received notification from the Department for Environment, Food and Rural Affairs (DEFRA) confirming that two dead wild birds have tested positive for avian influenza (bird flu).
The birds were found at:
We have been in contact with…

Cholelithiasis with concomitant choledocholithiasis is a frequently encountered condition in hepatobiliary surgery. Its incidence has increased in recent years, partly due to improved life expectancy and lifestyle change. In some…

THE ECONOMIC burden of diabetes is far greater than previously recognised and is set to place sustained pressure on health systems and societies worldwide, according to a large global modelling study spanning 204 countries and…

Christmas and New Year gatherings may have caused a bounce back in winter viruses, as the health service also grapples with a vicious cold snap.
Figures published today show that the number of patients in hospital beds with…
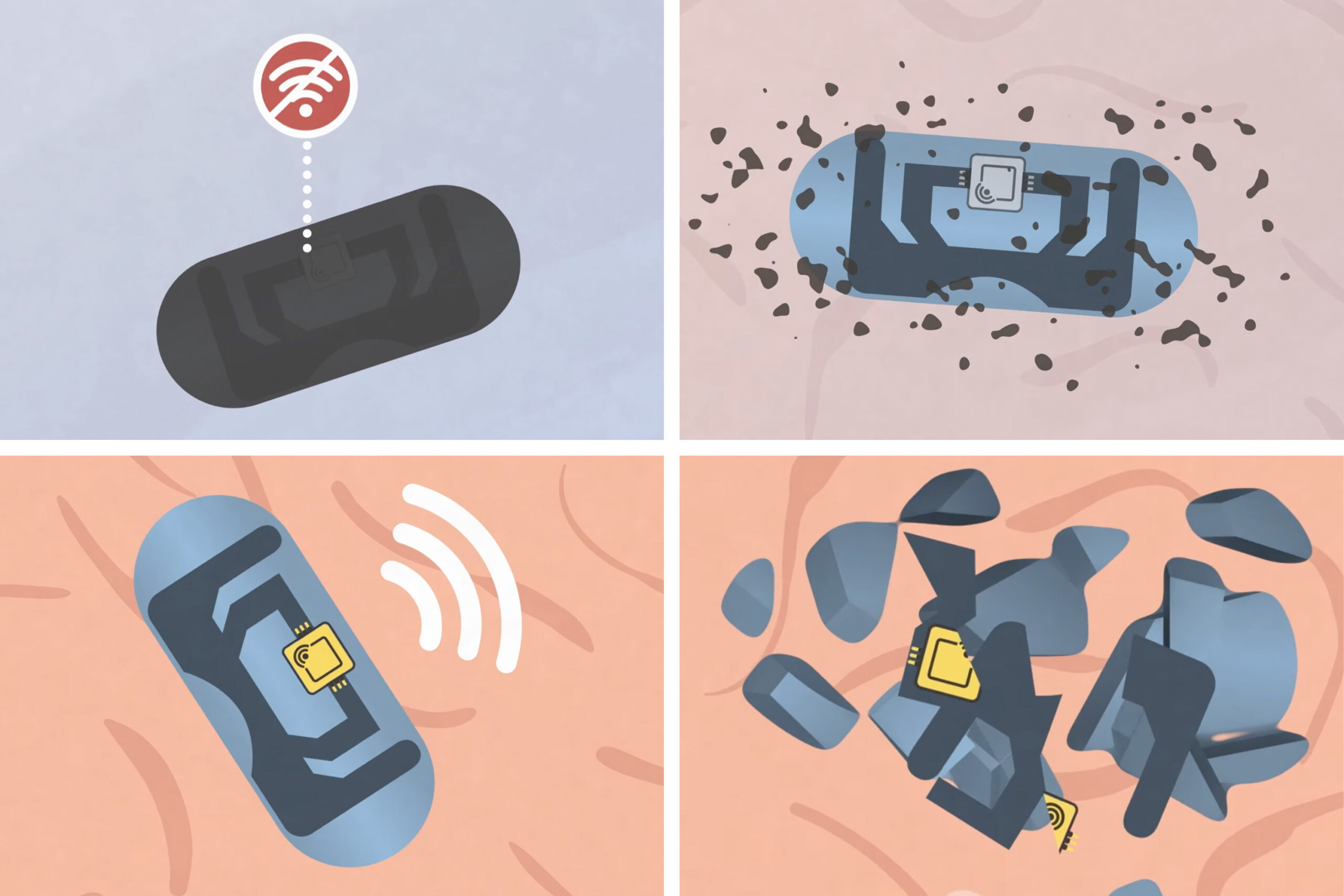
In an advance that could help ensure people are taking their medication on schedule, MIT engineers have designed a pill that can report when it has been swallowed.
The new reporting system, which can be incorporated into…
Published:
2026-01-08 09:29:00
January is globally observed as Cervical Cancer Awareness Month, a timely reminder of how preventable this disease can be with the right combination of vaccination and regular screening. Cervical cancer develops slowly and is most commonly caused…

As people return to gyms or start new fitness routines in the new year, new research suggests that even a short burst of intense exercise could play a role in protecting against cancer. Scientists report that as little as 10 minutes of hard…