Depienne, C. & Mandel, J.-L. 30 years of repeat expansion disorders: what have we learned and what are the remaining challenges? Am. J. Hum. Genet. 108, 764–785 (2021).
…
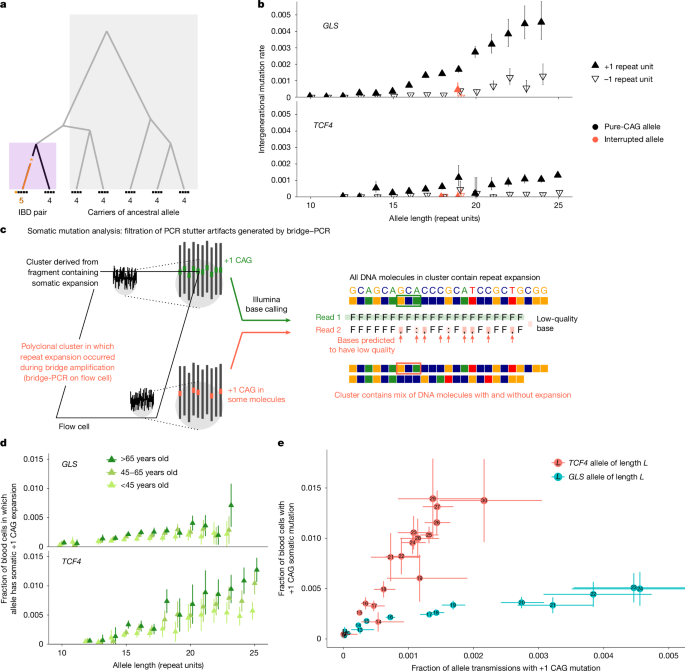
Depienne, C. & Mandel, J.-L. 30 years of repeat expansion disorders: what have we learned and what are the remaining challenges? Am. J. Hum. Genet. 108, 764–785 (2021).
…

Screening for elevated levels of lipoprotein (Lp)a levels among healthy women may be warranted, say researchers after finding that extremely high levels were associated with long-term…
Health
Research suggests even brief episodes of heavy alcohol consumption can injure small intestine
A new study shows that a single drinking binge…

The NHS in the South West is urging people to play their part as staff work flat-out to cater for high demand in the continuing cold weather.
With the amber weather alert extended to the weekend, there is heavy pressure…

Artificial Intelligence (AI) has emerged as a transformative force in healthcare, offering unprecedented opportunities to enhance diagnostic accuracy, personalize treatment protocols, and optimize clinical workflows.1,2 From…

According to the World Health Organization, in 2022, 2.3 million women were diagnosed with breast cancer globally. Breast cancer is the most common cancer among women in 157 countries, and women in every country, at any age after…
Nova Scotia Health’s mobile primary care clinics have upcoming dates in Dartmouth and Lower Sackville:
Dartmouth:
Dartmouth South Primary Care Clinic
380 Portland Street, Dartmouth
Saturday, January 10 from 9 a.m. to 4 p.m.
Sunday, January 11 from 9…
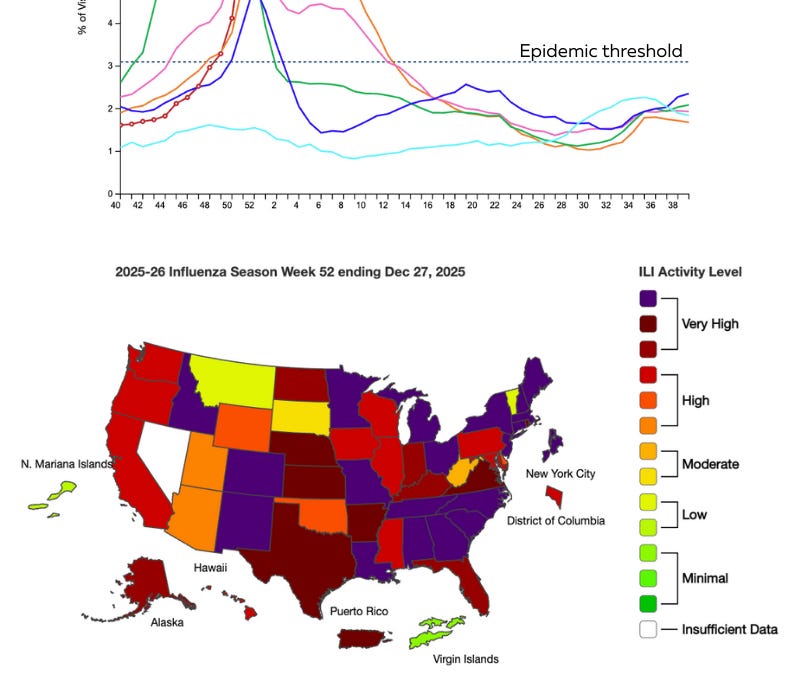
Well, it’s been a week. But happy New Year! I hope it was peaceful, magical, and restful. If you’re a parent, I’m guessing you’re just as excited as I am that school is back in session.
There’s one big thing we need to talk about: flu….

Ovarian mature cystic teratomas (MCTs) are widely known as germ cell-derived neoplasms capable of multilineage differentiation.1 Although generally benign, these tumors exhibit malignant transformation in approximately 1–2% of…