Vasdeki D, Tsamos G, Dimakakos E, Patriarcheas V, Koufakis T, Kotsa K, et al. Vitamin D Supplementation: Shedding Light on the Role of the Sunshine Vitamin in the Prevention and Management of Type 2 Diabetes and Its Complications. Nutrients. 2024;16(21):3651.
Article
CAS
PubMed Central
Google Scholar
Lhamo Y, Chugh PK, Tripathi C. Vitamin D supplements in the Indian market. Indian J Pharm Sci. 2016;78(1):41.
Article
CAS
PubMed Central
Google Scholar
Kittaneh M, Qurt M, Malkieh N, Naseef H, Muqedi R. Preparation and evaluation of vitamin D3 supplementation as transdermal film-forming solution. Pharmaceutics. 2022;15(1):39.
Article
PubMed Central
Google Scholar
Soubgui AF, Mboussi WS, Foko LP, Enyegue EL, Mogtomo ML. Exploring demographical, clinical, and dietary determinants of vitamin D deficiency among adults in Douala, Cameroon during the COVID-19 era. Heliyon. 2024;10(3).
Shaka MF, Meshesha MD, Borde MT. Vitamin D deficiency among apparently healthy children and children with common medical illnesses in Sub-Saharan Africa: a systematic review and meta-analysis. Ann Med Surg. 2022;75.
Woolley IJ, Giles ML, Howard JE, Korman TM. Unrecognised vitamin D deficiency: low concentrations in African migrants with HIV in Australia. Sexual Health. 2008;5(4):375–6.
Article
Google Scholar
Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review. Lancet Diabetes Endocrinol. 2014;2(1):76–89.
Article
CAS
Google Scholar
Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nat Rev Cancer. 2014;14(5):342–57.
Article
CAS
Google Scholar
Veldman CM, Cantorna MT, DeLuca HF. Expression of 1, 25-dihydroxyvitamin D3 receptor in the immune system. Arch Biochem Biophys. 2000;374(2):334–8.
Article
CAS
Google Scholar
Di Rosa M, Malaguarnera M, Nicoletti F, Malaguarnera L. Vitamin D3: a helpful immuno-modulator. Immunology. 2011;134(2):123–39.
Article
PubMed Central
Google Scholar
Ross AC, Taylor CL, Yaktine AL, Del Valle HB. Committee to review dietary reference intakes for vitamin D and calcium. Food and Nutrition Board. 2011;22:35–111.
Google Scholar
Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6(11):847–58.
Article
CAS
Google Scholar
Kozłowska J, Kiełt W, Broniec G, Wajdowicz B, Kudła A, Czapiewska R, et al. The significance of Vitamin D in dentistry-rewiew. Journal of Education, Health and Sport. 2024;67:55046-.
Bouillon R, LeBoff MS, Neale RE. Health effects of vitamin D supplementation: lessons learned from randomized controlled trials and mendelian randomization studies. J Bone Miner Res. 2023;38(10):1391–403.
Article
Google Scholar
Engin MMN, Özdemir Ö. Role of vitamin D in COVID-19 and other viral infections. World Journal of Virology. 2024;13(3):95349.
Article
PubMed Central
Google Scholar
AlGhamdi SA. Effectiveness of Vitamin D on Neurological and Mental Disorders. Diseases. 2024;12(6):131.
Article
CAS
PubMed Central
Google Scholar
Ruiz-Garcia A, Pallares-Carratala V, Turegano-Yedro M, Torres F, Sapena V, Martin-Gorgojo A, et al. Vitamin D supplementation and its impact on mortality and cardiovascular outcomes: systematic review and meta-analysis of 80 randomized clinical trials. Nutrients. 2023;15(8):1810.
Article
CAS
PubMed Central
Google Scholar
Newberry SJ, Chung M, Shekelle PG, Booth MS, Liu JL, Maher AR, et al. Vitamin D and calcium: a systematic review of health outcomes (update). Evid Rep Technol Assess. 2014;217:1–929.
Google Scholar
Wimalawansa SJ, Weiss ST, Hollis BW. Integrating Endocrine, Genomic, and Extra-Skeletal Benefits of Vitamin D into National and Regional Clinical Guidelines. Nutrients. 2024;16(22):3969.
Article
CAS
PubMed Central
Google Scholar
Giustina A, Bilezikian JP, Adler RA, Banfi G, Bikle DD, Binkley NC, et al. Consensus statement on Vitamin D status assessment and supplementation: whys, whens, and hows. Endocrine Reviews. 2024:bnae009.
Whiting SJ, Calvo MS. Vitamin D supplement use as a public health strategy to augment diet and sustain population adequacy. Feldman and Pike’s Vitamin D: Elsevier; 2024. p. 115–33.
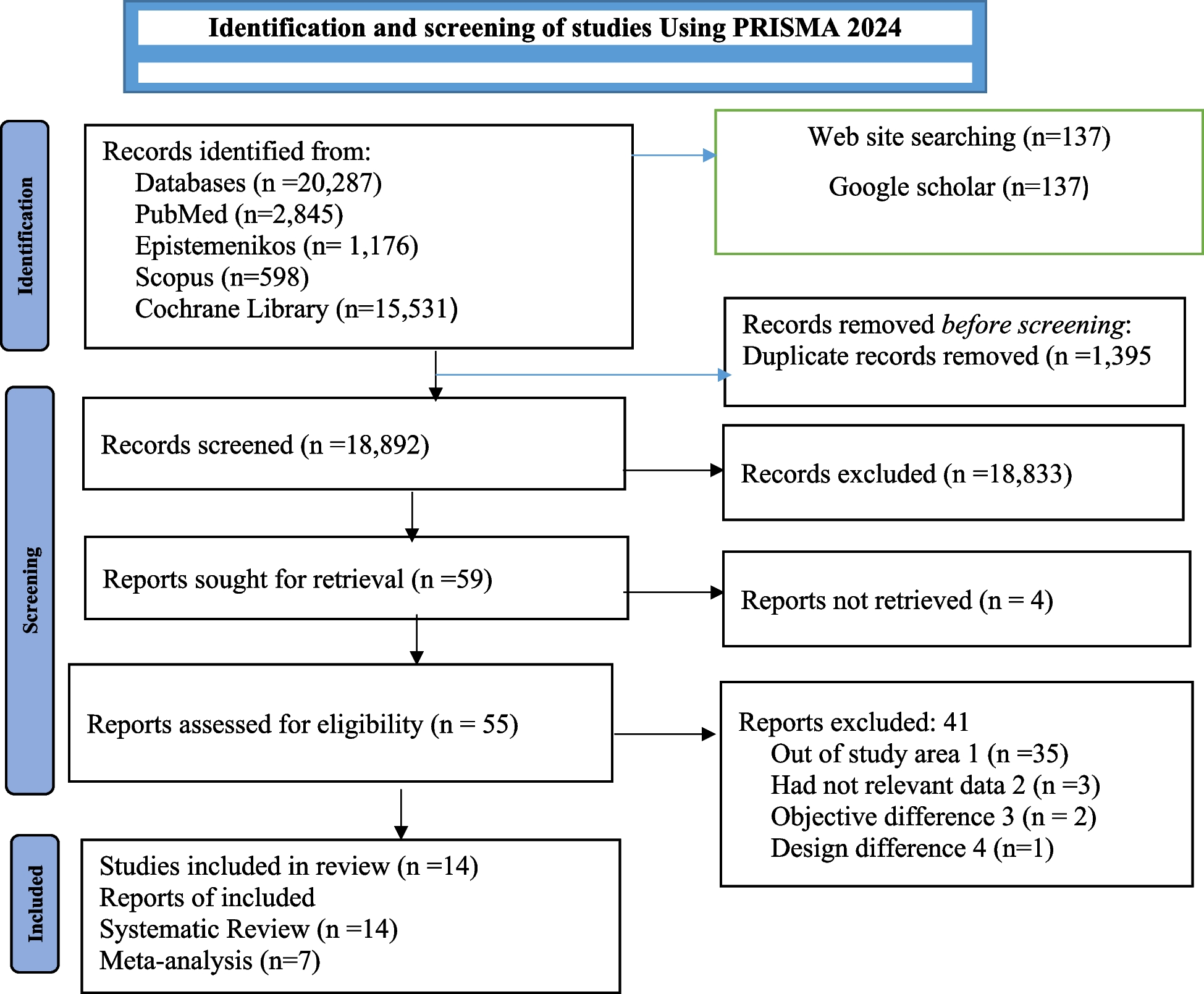
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. bmj. 2021;372.
Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane handbook for systematic reviews of interventions. 2019:205–28.
Handschuh C, Navarra A-M. Appraise and synthesize the evidence. Evidence-based practice improvement: merging evidence-based practice and quality improvement. 2024:163.
Swartz MK. PRISMA 2020: an update. J Pediatr Health Care. 2021Jul 1;35(4):351.
Article
Google Scholar
Middelkoop K, Micklesfield L, Stewart J, Walker N, Jolliffe DA, Mendham AE, et al. Influence of vitamin D supplementation on growth, body composition, pubertal development and spirometry in South African schoolchildren: a randomised controlled trial (ViDiKids). BMJ Paediatrics Open. 2024;8(1): e002495.
Article
PubMed Central
Google Scholar
Middelkoop K, Micklesfield L, Hemmings S, Walker N, Stewart J, Jolliffe DA, Mendham AE, Tang JC, Cooper C, Harvey NC, Wilkinson RJ. Influence of vitamin D supplementation on muscle strength and exercise capacity in South African schoolchildren: secondary outcomes from a randomised controlled trial (ViDiKids). BMJ Open Sport Exerc Med. 2024;10(3).
Middelkoop K, Stewart J, Walker N, Delport C, Jolliffe DA, Coussens AK, et al. Vitamin D supplementation to prevent tuberculosis infection in South African schoolchildren: multicenter phase 3 double-blind randomized placebo-controlled trial (ViDiKids). Int J Infect Dis. 2023;134:63–70.
Article
CAS
Google Scholar
Middelkoop K, Micklesfield LK, Walker N, Stewart J, Delport C, Jolliffe DA, et al. Influence of vitamin D supplementation on bone mineral content, bone turnover markers, and fracture risk in South African schoolchildren: multicenter double-blind randomized placebo-controlled trial (ViDiKids). J Bone Miner Res. 2024;39(3):211–21.
Article
PubMed Central
Google Scholar
Hassan RHA, Bahe SMAE, Mohamed AIZ, Sakoury MMA, Akl HF, Ababtain HAS, et al. The effect of high-dose vitamin D supplementation and an exercise program to lose weight on some biochemical variables of overweight women. Pedagogy Physical Culture Sports. 2023;27(5):353–60.
Article
Google Scholar
Gad AI, Elmedames MR, Abdelhai AR, Marei AM, Abdel-Ghani HA. Efficacy of vitamin D supplementation on adult patients with non-alcoholic fatty liver disease: a single-center experience. Gastroenterology and Hepatology From Bed to Bench. 2021;14(1):44.
PubMed Central
Google Scholar
Ashenafi S, Amogne W, Kassa E, Gebreselassie N, Bekele A, Aseffa G, et al. Daily Nutritional Supplementation with Vitamin D3 and Phenylbutyrate to Treatment-Naïve HIV Patients Tested in a Randomized Placebo-Controlled Trial. Nutrients. 2019;11(1):133.
Article
CAS
PubMed Central
Google Scholar
Akrour-Aissou C, Dupré T, Grangaud JP, Assami MK. Impact of vitamin D supplementation model on the circulating levels of 25 (OH) D in Algerian children aged 1–23 months. J Steroid Biochem Mol Biol. 2020;196: 105487.
Article
CAS
Google Scholar
Steenhoff AP, Schall JI, Samuel J, Seme B, Marape M, Ratshaa B, et al. Vitamin D₃ supplementation in Batswana children and adults with HIV: a pilot double blind randomized controlled trial. PLoS One. 2015;10(2): e0117123.
Article
PubMed Central
Google Scholar
Abroug H, Maatouk A, Bennasrallah C, Dhouib W, Ben Fredj M, Zemni I, et al. Effect of vitamin D supplementation versus placebo on recovery delay among COVID-19 Tunisian patients: a randomized-controlled clinical trial. Trials. 2023;24(1):123.
Article
CAS
PubMed Central
Google Scholar
Wejse C, Gomes VF, Rabna P, Gustafson P, Aaby P, Lisse IM, et al. Vitamin D as supplementary treatment for tuberculosis: a double-blind, randomized, placebo-controlled trial. Am J Respir Crit Care Med. 2009;179(9):843–50.
Article
CAS
Google Scholar
Elfituri S. The effects of vitamin D supplementation on disease activity and fatigue in Libyan rheumatoid arthritis patients. Reumatologia. 2024;62(2):109.
Article
PubMed Central
Google Scholar
Muhihi A, Fawzi WW, Aboud S, Nagu TJ, Ulenga N, Wang M, et al. Cholecalciferol Supplementation Does Not Affect the Risk of HIV Progression, Viral Suppression, Comorbidities, Weight Loss, and Depression among Tanzanian Adults Initiating Antiretroviral Therapy: Secondary Outcomes of a Randomized Trial. J Nutr. 2022;152(8):1983–90.
Article
PubMed Central
Google Scholar
Sudfeld CR, Mugusi F, Muhihi A, Aboud S, Nagu TJ, Ulenga N, et al. Efficacy of vitamin D3 supplementation for the prevention of pulmonary tuberculosis and mortality in HIV: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2020;7(7):e463–71.
Article
PubMed Central
Google Scholar
Margolis KL, Lurie N, McGovern PG, Tyrrell M, Slater JS. Increasing breast and cervical cancer screening in low-income women. J Gen Intern Med. 1998;13(8):515–21.
Article
CAS
PubMed Central
Google Scholar
Charoenngam N. Vitamin D and rheumatic diseases: a review of clinical evidence. Int J Mol Sci. 2021;22(19):10659.
Article
CAS
PubMed Central
Google Scholar
Cutolo M, Otsa K, Uprus M, Paolino S, Seriolo B. Vitamin D in rheumatoid arthritis. Autoimmun Rev. 2007;7(1):59–64.
Article
CAS
Google Scholar
Guan Y, Hao Y, Guan Y, Bu H, Wang H. The effect of vitamin D supplementation on rheumatoid arthritis patients: a systematic review and meta-analysis. Front Med. 2020;7: 596007.
Article
Google Scholar
Kitson MT, Roberts SK. D-livering the message: the importance of vitamin D status in chronic liver disease. J Hepatol. 2012;57(4):897–909.
Article
CAS
Google Scholar
Hussain M, Iqbal J, Malik SA, Waheed A, Shabnum S, Akhtar L, Saeed H. Effect of vitamin D supplementation on various parameters in non-alcoholic fatty liver disease patients. Pak J Pharm Sci. 2019;32.
Radwan AM, Tawfik MA, Nagy HM, Kotb NA. Effect of vitamin D replacement therapy on laboratory parameters in hepatitis C virus cirrhotic patients. Tanta Med J. 2022;50(4):260–6.
Article
Google Scholar
Ganmaa D, Hemmings S, Jolliffe DA, Buyanjargal U, Garmaa G, Adiya U, et al. Influence of vitamin D supplementation on muscle strength and exercise capacity in Mongolian schoolchildren: secondary outcomes from a randomised controlled trial. BMJ Open Sport Exercise Med. 2024;10(3).
Chen X, Zhao Y, Zhang R, Zhao Y, Dai L. The effect of vitamin D supplementation on some metabolic parameters in patients with nonalcoholic fatty liver disease: A systematic review and meta-analysis of 8 RCTs. Medicine. 2023;102(42): e35717.
Article
CAS
PubMed Central
Google Scholar
Lundblad M, Waldén M, Magnusson H, Karlsson J, Ekstrand J. The UEFA injury study: 11-year data concerning 346 MCL injuries and time to return to play. Br J Sports Med. 2013;47(12):759–62.
Article
Google Scholar
Janssens W, Bouillon R, Claes B, Carremans C, Lehouck A, Buysschaert I, et al. Vitamin D deficiency is highly prevalent in COPD and correlates with variants in the vitamin D-binding gene. Thorax. 2010;65(3):215–20.
Article
Google Scholar
Lange NE, Sparrow D, Vokonas P, Litonjua AA. Vitamin D deficiency, smoking, and lung function in the Normative Aging Study. Am J Respir Crit Care Med. 2012;186(7):616–21.
Article
CAS
PubMed Central
Google Scholar
Bassatne A, Basbous M, Chakhtoura M, El Zein O, Rahme M, Fuleihan GE-H. The link between COVID-19 and VItamin D (VIVID): A systematic review and meta-analysis. Metabolism. 2021;119:154753.
Entrenas Castillo M, Entrenas Costa L, Vaquero Barrios J, Alcalá Díaz J, López Miranda J, Bouillon R, et al. Effect of calcifediol treatment and best available therapy versus best available therapy on intensive care unit 36 William B. Grant; 2020.
Bjelakovic G, Gluud LL, Nikolova D, Whitfield K, Krstic G, Wetterslev J, Gluud C. Vitamin D supplementation for prevention of cancer in adults. Cochrane Database Syst Rev. 2014;(6).
Chowdhury R, Kunutsor S, Vitezova A, Oliver-Williams C, Chowdhury S, Kiefte-de-Jong JC, Khan H, Baena CP, Prabhakaran D, Hoshen MB, Feldman BS. Vitamin D and risk of cause specific death: systematic review and meta-analysis of observational cohort and randomised intervention studies. Bmj. 2014;348.
Wilson BM, Ross RD, Jacobs JJ, Sumner DR. Comparison of Bone Turnover Biomarkers in Serum and Urine Measured on an Automated Analytical Platform. J Appl Lab Med. 2021;6(3):750–5.
Article
Google Scholar
Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, et al. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–8.
Article
CAS
Google Scholar
Viard J-P, Souberbielle J-C, Kirk O, Reekie J, Knysz B, Losso M, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. AIDS. 2011;25(10):1305–15.
Article
CAS
Google Scholar
Holick MF. Vitamin D: a d-lightful solution for health. J Investig Med. 2011;59(6):872–80.
Article
CAS
PubMed Central
Google Scholar
Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, et al. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103(4):1033–44.
Article
CAS
PubMed Central
Google Scholar
Viard J, Souberbielle J, Kirk O, Knysz B, Losso M, Gatell J, et al. Vitamin D and clinical disease progression in HIV infection: results from the EuroSIDA study. J Int AIDS Soc. 2010;13:1–2.
Article
Google Scholar
Holick M. Vitamin D, Deficiency. N Engl J Med. 2007;357(3):266–81. https://doi.org/10.1056/NEJMra070553.
Coussens AK, Naude CE, Goliath R, Chaplin G, Wilkinson RJ, Jablonski NG. High-dose vitamin D3 reduces deficiency caused by low UVB exposure and limits HIV-1 replication in urban Southern Africans. Proc Natl Acad Sci. 2015;112(26):8052–7.
Article
CAS
PubMed Central
Google Scholar
Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–51.
Article
Google Scholar
Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–6.
Article
CAS
PubMed Central
Google Scholar
Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988.
Article
CAS
PubMed Central
Google Scholar


 Epidemiology team from the state of Rio de Janeiro using Go.Data in response to an outbreak [2025]. © Pan American Health Organization, Brazil.
Epidemiology team from the state of Rio de Janeiro using Go.Data in response to an outbreak [2025]. © Pan American Health Organization, Brazil.