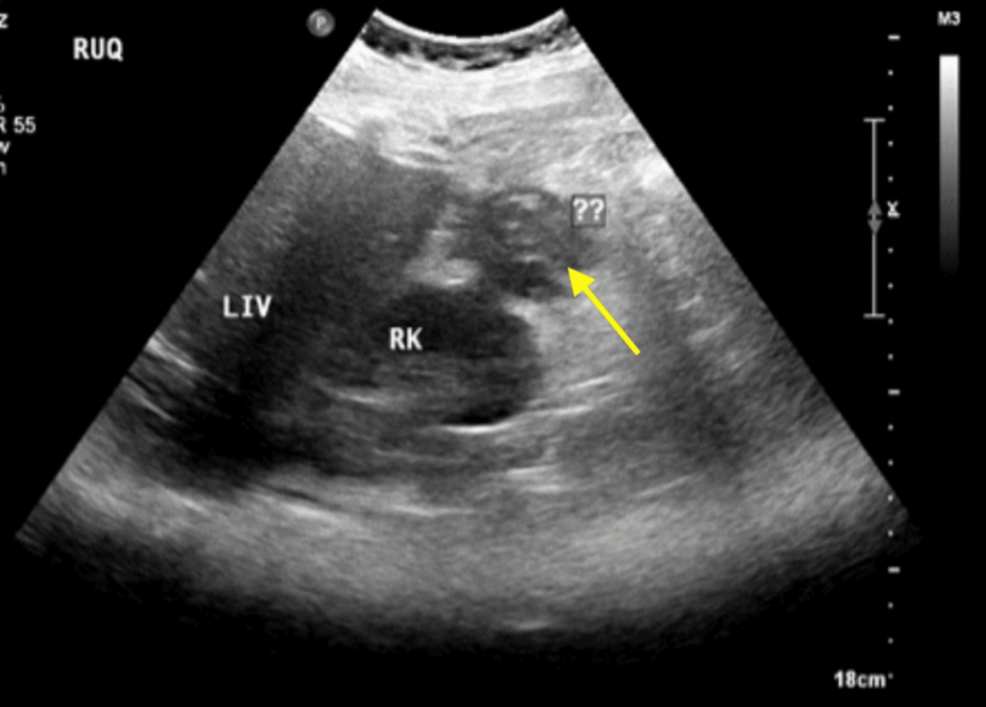
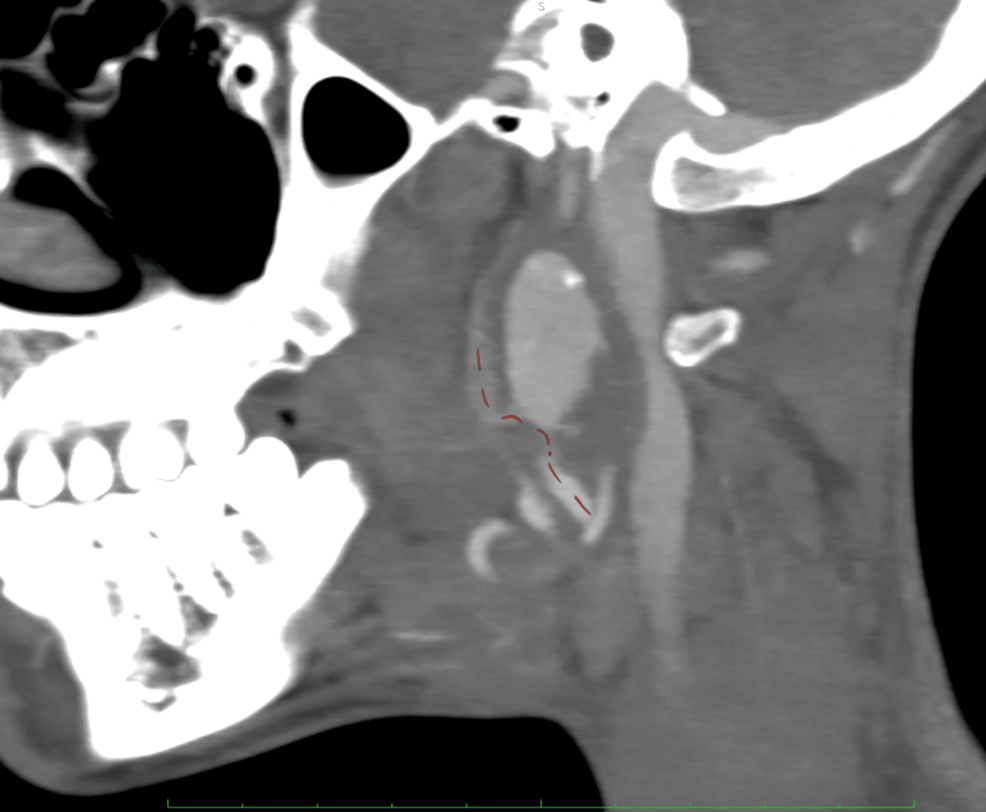
BEIJING, Jan. 7 (Xinhua) — A team of Chinese scientists has demonstrated that simple, non-invasive sound therapy can produce significant and remarkably long-lasting biological changes in aged monkeys, offering new hope for a potential…
Category: 6. Health
-
New research reveals Iron deficiency could lead to Parkinson’s disease
Iron is one of the most essential minerals for your body however, did you…
Continue Reading
-

Infectious diseases worsen in Pakistan; alarming deaths revealed
A government household survey has revealed a deepening public health crisis in Pakistan, where thousands of people die every year from preventable infectious diseases.
The Household Economic Survey has uncovered…
Continue Reading
-

GSK’s Shingrix (Recombinant Zoster Vaccine) prefilled syringe presentation approved by the European Commission
- Prefilled syringe offers healthcare professionals a convenient administration option
- New presentation will begin rolling out across EU countries in 2026
- Shingles affects approximately 1.7 million people in Europe each year,1 with certain chronic…
Continue Reading
-

A second chance for first place at the British Transplant Games – Positive News
Behind every medal at the British Transplant Games is a story of survival and generosity. Athletes are competing not just for themselves, but in honour of the donors who gave them a second…
Continue Reading
-

Future Oncology at a glance: a spotlight on lung cancer
Discover a curated selection of lung cancer articles published in Future Oncology in this 2025 roundup, which highlights key content pieces and their potential clinical impact. The value of real-world evidence in supporting targeted…
Continue Reading
-
Tool predicts mental health and treatment needs for new inflammatory bowel disease patients
The IBD Disk is used in clinical practice to help patients with IBD track how their condition affects different aspects of daily life, such as energy levels, sleep, and emotional wellbeing.
The Birmingham study is the first to show that the tool…
Continue Reading
-
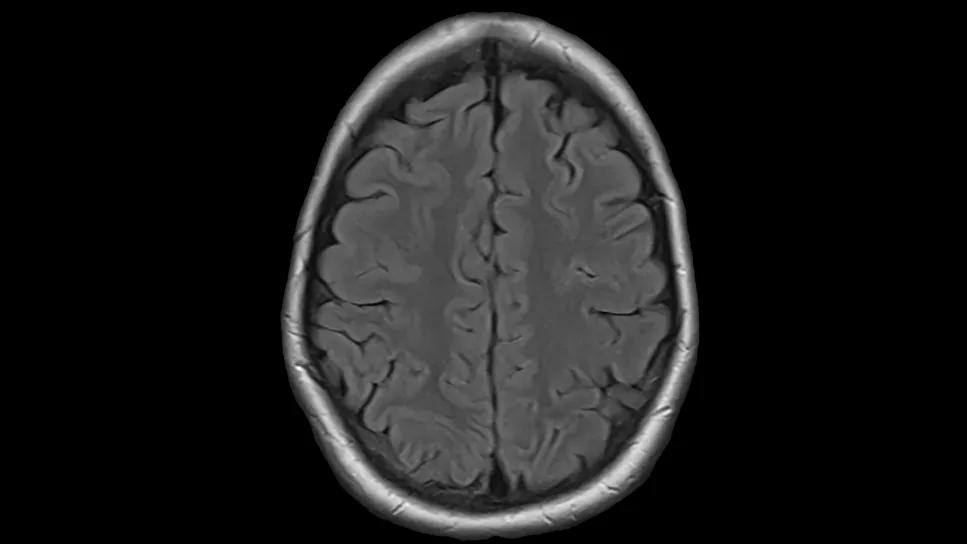
21-Year-Old Patient with Refractory T-Cell Lymphoma
A 21-year-old patient with aggressive lymphoma sought care at Cleveland Clinic Cancer Institute after rapid acceleration of her disease. Caring for a young patient with lymphoma and swift progression to central nervous system (CNS) disease…
Continue Reading