James GallagherHealth and science correspondent
 BBC
BBCBowel cancer samples that have been stored for up to a century will be analysed to try to solve the mysterious rise of the disease in young…

James GallagherHealth and science correspondent
 BBC
BBCBowel cancer samples that have been stored for up to a century will be analysed to try to solve the mysterious rise of the disease in young…

Holly, 27, is one of a growing number of young people getting the disease.
Her bloating and weight loss were originally put down to irritable bowel syndrome until she became so ill she ended up in A&E.
The young actress was diagnosed with advanced…

Janine MachinEast of England technology correspondent
 BBC
BBCIn a dark room on Cambridge Science Park, two humanoid robots wearing trainers are walking…

Hyaluronic acid (HA) dermal fillers are widely utilized for facial rejuvenation, volumization, and skin quality enhancements in non-invasive aesthetic procedures, owing to their favorable biocompatibility and reversibility.1 As a…

Guarnieri, G. et al. Contemporary management of acute pulmonary embolism. Int. J. Cardiol. 436, 133422 (2025).
Smulders, Y. M. Pathophysiology and treatment of haemodynamic instability in acute…

Many people focus on drinking enough water daily, but often overlook its temperature. Health experts note that drinking hot water early in the morning can offer multiple benefits for digestion, circulation, and…
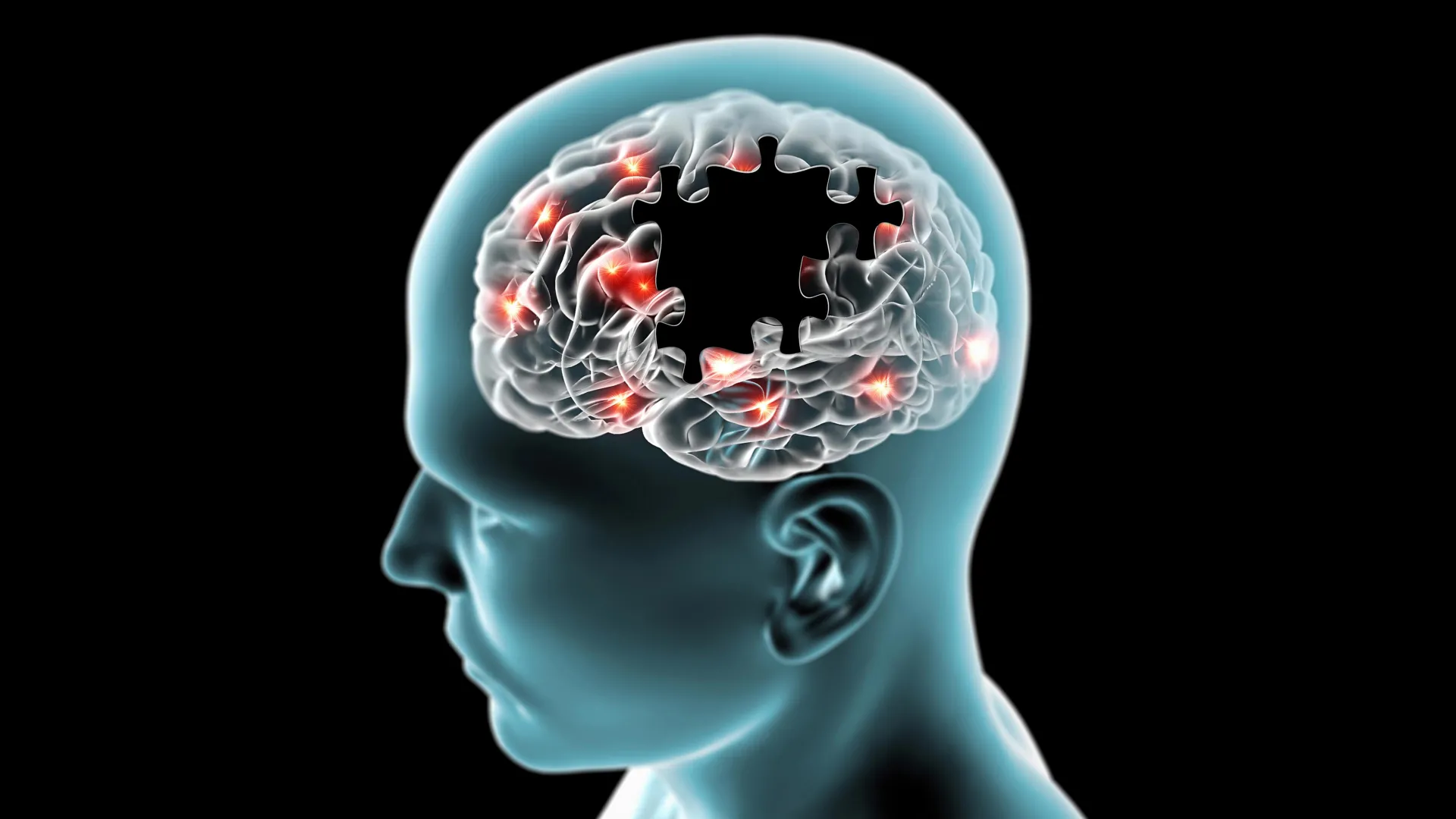
There is a new reason to take daily tooth brushing seriously. Researchers in South Korea have found strong evidence that bacteria from the mouth can move into the gut and influence brain cells, potentially playing a role in the development of…
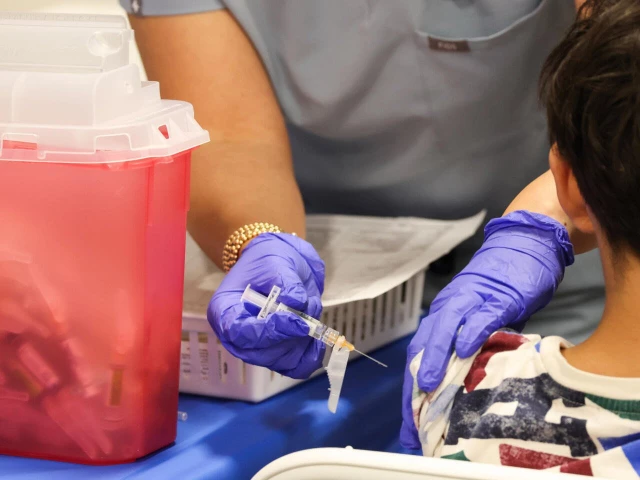
All vaccines currently recommended by CDC will remain covered by insurance without cost sharing
The US will no longer recommend that every child receive immunizations against several diseases including rotavirus and influenza. Photo: AFP (file)
In a revealing Genomic Press Interview published today in Genomic Psychiatry, Dr. Maria Margarita Behrens recounts an extraordinary scientific journey that wound through four countries and multiple disciplines before arriving at…