WASHINGTON — The U.S. took the unprecedented step Monday of cutting the number of vaccines it recommends for every child — a move that leading medical groups said would undermine protections against a half-dozen diseases.
The change is…

WASHINGTON — The U.S. took the unprecedented step Monday of cutting the number of vaccines it recommends for every child — a move that leading medical groups said would undermine protections against a half-dozen diseases.
The change is…


For patients with compensated metabolic dysfunction-associated steatohepatitis (MASH) cirrhosis and obesity, the clinical path is fraught with complexity. These patients face a “dual burden”—progressive liver disease compounded by severe…
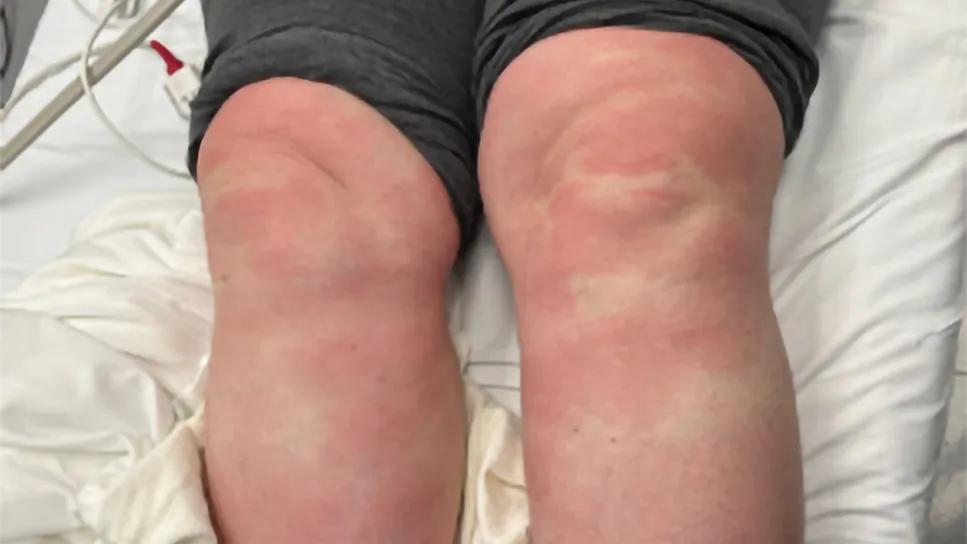
A 53-year-old female presented to the hospital with a history of persistent fever. Approximately three weeks before she was admitted, she began experiencing fevers ranging from 100° to 102°F, accompanied by chills and myalgias. These fevers…

Ageing is inevitable, but losing your mental sharpness doesn’t have to be part of the deal.
While decline is often treated as an unavoidable consequence of growing older, growing evidence suggests that everyday habits play a powerful role in…

New regulations come into force on Monday in Britain banning daytime TV and online adverts for so-called junk foods, in what the government calls a “world-leading action” to tackle childhood obesity.
The ban – targeting ads for products…

Cerebral infarction is the second leading cause of death globally and the leading cause of death in China.1 Ischemic stroke is the predominant type, accounting for over 80% of all strokes. It occurs when a major cerebral artery is…
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Aune, D. et al. BMI and all cause mortality: systematic review…

Despite a recent progress in the diversity of treatment options, lung cancer remains the leading cause of cancer-related deaths worldwide, with an estimated annual number of around 125,000 cancer deaths in the US alone.1 Small cell…