Gerrity, D., Crank, K., Steinle-Darling, E. & Pecson, B. M. Establishing pathogen log reduction value targets for direct potable reuse in the united States. AWWA Water Sci. 5, e1353 (2023).
Gerrity, D., Crank, K., Steinle-Darling, E. & Pecson, B. M. Establishing pathogen log reduction value targets for direct potable reuse in the united States. AWWA Water Sci. 5, e1353 (2023).
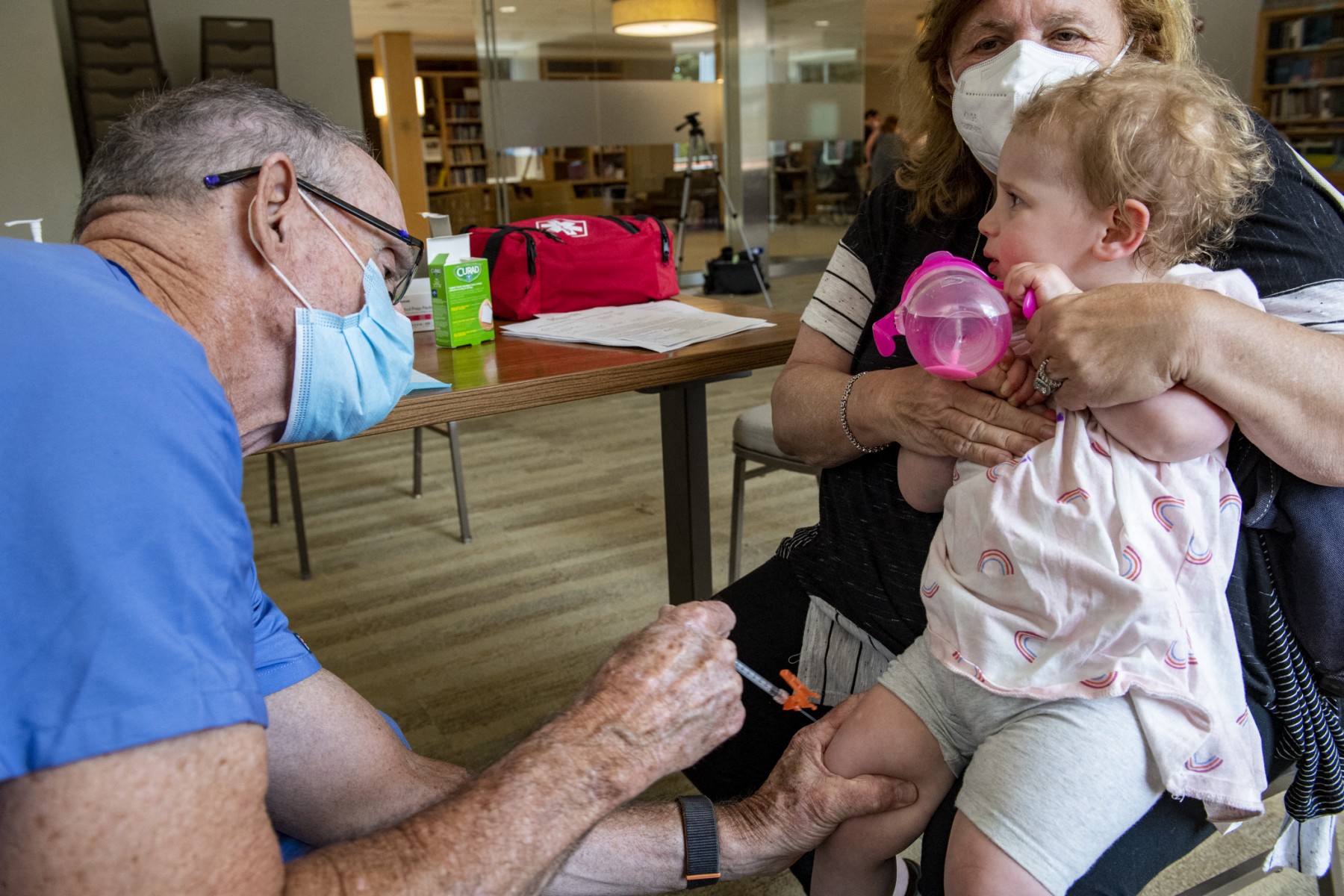
Leading medical groups in the United States have raised alarm after the Centers for Disease Control and Prevention (CDC) under President Donald Trump took the unprecedented step of cutting the number of vaccines it recommends for…

Nick TriggleHealth correspondent
 Getty Images
Getty ImagesThe new NHS online hospital service being launched in England next year will initially focus on menopause, prostate and eye conditions.
The NHS has selected nine different conditions in total for the…
Infectious disease researchers have identified Pteropine orthoreoviruses (PRVs), a group of newly emergent bat-borne viruses, as the culprit for previously unexplained illness in five Bangladeshi patients, one of whom eventually…
Welcome to Day 2 of the Brain Health Challenge. Today, we’re talking about food.
Your brain is an energy hog. Despite comprising about 2 percent of the average person’s body mass, it consumes roughly 20 percent of the body’s energy. In…

A large international modelling study reveals that the hidden cost of unpaid caregiving, not healthcare alone, drives the staggering long-term economic impact of diabetes across countries and income levels.
Study: The global…

Interviewer: How to eat to control your cholesterol. Sharee Thompson is a registered dietitian nutritionist at University of Utah. Sharee, first of all, when it comes to helping…
Meena, S., Sharma, P., Sambharia, A. K. & Dawar, A. Fractures of distal radius: an overview. J. Family Med. Prim. Care. 3 (4), 325–332 (2014).
Çelik, M. et al. The effects of phenyramidol…