A large international modelling study reveals that the hidden cost of unpaid caregiving, not healthcare alone, drives the staggering long-term economic impact of diabetes across countries and income levels.
Study: The global…

A large international modelling study reveals that the hidden cost of unpaid caregiving, not healthcare alone, drives the staggering long-term economic impact of diabetes across countries and income levels.
Study: The global…

Interviewer: How to eat to control your cholesterol. Sharee Thompson is a registered dietitian nutritionist at University of Utah. Sharee, first of all, when it comes to helping…
Meena, S., Sharma, P., Sambharia, A. K. & Dawar, A. Fractures of distal radius: an overview. J. Family Med. Prim. Care. 3 (4), 325–332 (2014).
Çelik, M. et al. The effects of phenyramidol…
World Health Organization. Global hepatitis report 2024: action for access in low- and middle-income countries, <https://www.who.int/publications/i/item/9789240091672> (2024).
Pattyn, J., Hendrickx, G., Vorsters, A. & Van Damme, P. Hepatitis B…
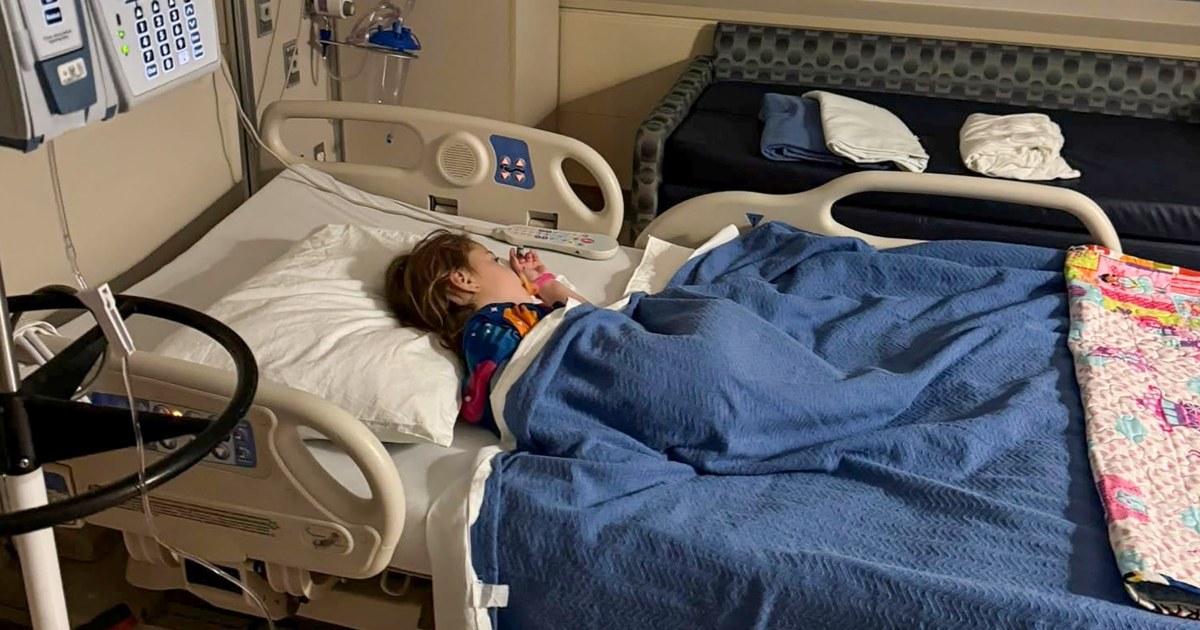
Doctors’ visits for flu-like symptoms — fevers, sore throat, extreme fatigue and body aches — have hit the highest level in nearly 30 years, according to the Centers for Disease Control and Prevention, and are likely to continue to rise in…
![[Press release] WHO releases updated recommendations on HIV clinical management](https://afnnews.qaasid.com/wp-content/uploads/2025/10/doctor-g67f140729-640.png)
05 January 2026 | Geneva — The World Health Organization (WHO) has released updated recommendations on HIV clinical management providing new and revised guidance on antiretroviral therapy, management of vertical HIV…

The Immunotherapy and Precision Oncology Forum (IPOF 2026) is the 5th annual hybrid conference scheduled for Saturday, April 11, 2026, in West Chester, Ohio. Presented by the Innovative Healthcare Institute and the Journal…