Category: 6. Health
-
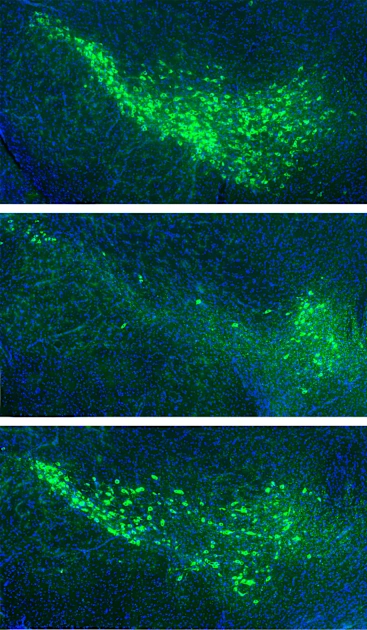
Researchers Identify Proteins that Spread Parkinson’s Pathology in the Brain
Neurodegenerative diseases like Alzheimer’s and Parkinson’s are a growing health concern in the United States. The Parkinson’s Foundation currently estimates that around 1.1 million Americans are diagnosed with Parkinson’s disease, and…
Continue Reading
-

There’s a huge loophole in the new UK ban on daytime junk food ads
New advertising restrictions on unhealthy food and drink have come into force in the UK, targeting products deemed to be high in fat, salt or sugar. From now on, TV, radio or online adverts that feature these foods will be banned before…
Continue Reading
-

what sunscreen ads reveal about beauty ideals and power
In the depths of winter, sunscreen might not be top of many people’s shopping lists. Yet it remains a staple in most households, and many of us are encouraged to use it year-round. But sunscreen’s history reveals more than just…
Continue Reading
-
Semaglutide Improves Knee Replacements for Diabetic Patients
The study found that even taking semaglutide for less than a month before surgery had benefits, such as fewer minor complications like wound issues, bleeding, kidney problems, pneumonia, and infections. Importantly, taking the medication for at…
Continue Reading
-
Celebrating 10 years of Nature Microbiology
Our January 2026 special issue commemorates the tenth anniversary of the journal and celebrates researchers, peer reviewers and readers from the microbiology community and beyond.
When Nature…
Continue Reading
-

Disabling prescribing system alerts may have led to hospital paracetamol overdose
Disabling decision-support alerts on a hospital prescribing system may have contributed to a patient’s death by paracetamol overdose, NHS England has suggested.
Paula Doreen Hughes, aged 55 years, died on 10 January 2022 following a medication…
Continue Reading
-

United Kingdom bans junk food ads
by Alimat Aliyeva
A new ban on advertising unhealthy food and drinks has come into
force in the UK as part of the government’s efforts to combat
childhood obesity, Azernews reports.Experts estimate that this measure…
Continue Reading
-

Machine Learning Predicts Hyperglycemia Risk in Psoriasis
Researchers have developed and validated a machine learning model using XGBoost to predict hyperglycemia risk in patients with
psoriasis .1 Tested on both clinical and National Health and Nutrition Examination Survey (NHANES) data sets, the model…Continue Reading
-

Calculator offers accurate scoring of multilingual language ability
More than half of the world’s population speaks more than one language-but there is no consistent method for defining “bilingual” or “multilingual.” This makes it difficult to accurately assess proficiency across multiple languages…
Continue Reading