HIV Reservoir Clones Remain the Key Cure Obstacle
A NEW study offers a rare window into HIV reservoir clones that can survive for life despite antiretroviral therapy (ART).
HIV latency, where the virus lies dormant in long-lived CD4+ T…
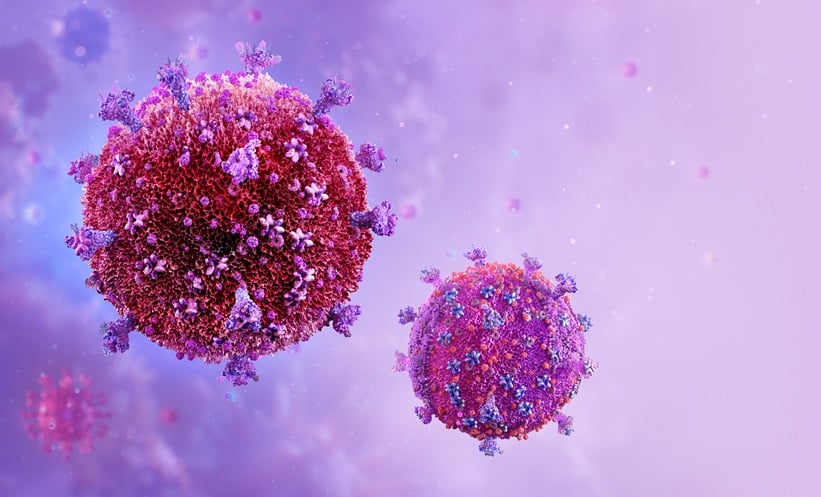
A NEW study offers a rare window into HIV reservoir clones that can survive for life despite antiretroviral therapy (ART).
HIV latency, where the virus lies dormant in long-lived CD4+ T…

CANCER after cardiac surgery was tied to shorter long-term survival, even though in-hospital mortality remained low.
As more patients present for cardiac surgery with active cancer or a prior…

Should people be encouraged to undertake an annual mental health check-up via AI or is that a bridge too far?
getty
In today’s column, I examine a somewhat novel idea that people should consider using generative AI and large language models as a…

When floods sweep through southern Africa, the most visible damage is immediate: homes washed away, crops destroyed, clinics disrupted, families displaced. These images dominate headlines and humanitarian appeals.
But as floodwaters recede,…
Schafer, E. J. et al. Recent Patterns and Trends in Global Prostate Cancer Incidence and Mortality. Update Eur. Urol. 87 (3), 302–313 (2025).
Van Blarigan, E. L. et al. Trends in Prostate…

Please describe your research?■ The SuperAgers study at Northwestern University was developed 25 years ago. Its goal is to examine individuals over the age of 80 who have the memory capacity of people aged 30 or 40 years younger. This aims to…

Men’s Fitness aims to feature only the best products and services. If you buy something via one of our links, we may earn a commission.
Meal timing has become nutrition’s favorite battlefield. Some swear by fasting windows, others by eating…