This series is based on our reporting on TCM: its history, treatments and growing acceptance around the world. This is the seventh instalment.
In the world of traditional Chinese medicine (TCM), few remedies carry the legendary status – or the…

This series is based on our reporting on TCM: its history, treatments and growing acceptance around the world. This is the seventh instalment.
In the world of traditional Chinese medicine (TCM), few remedies carry the legendary status – or the…
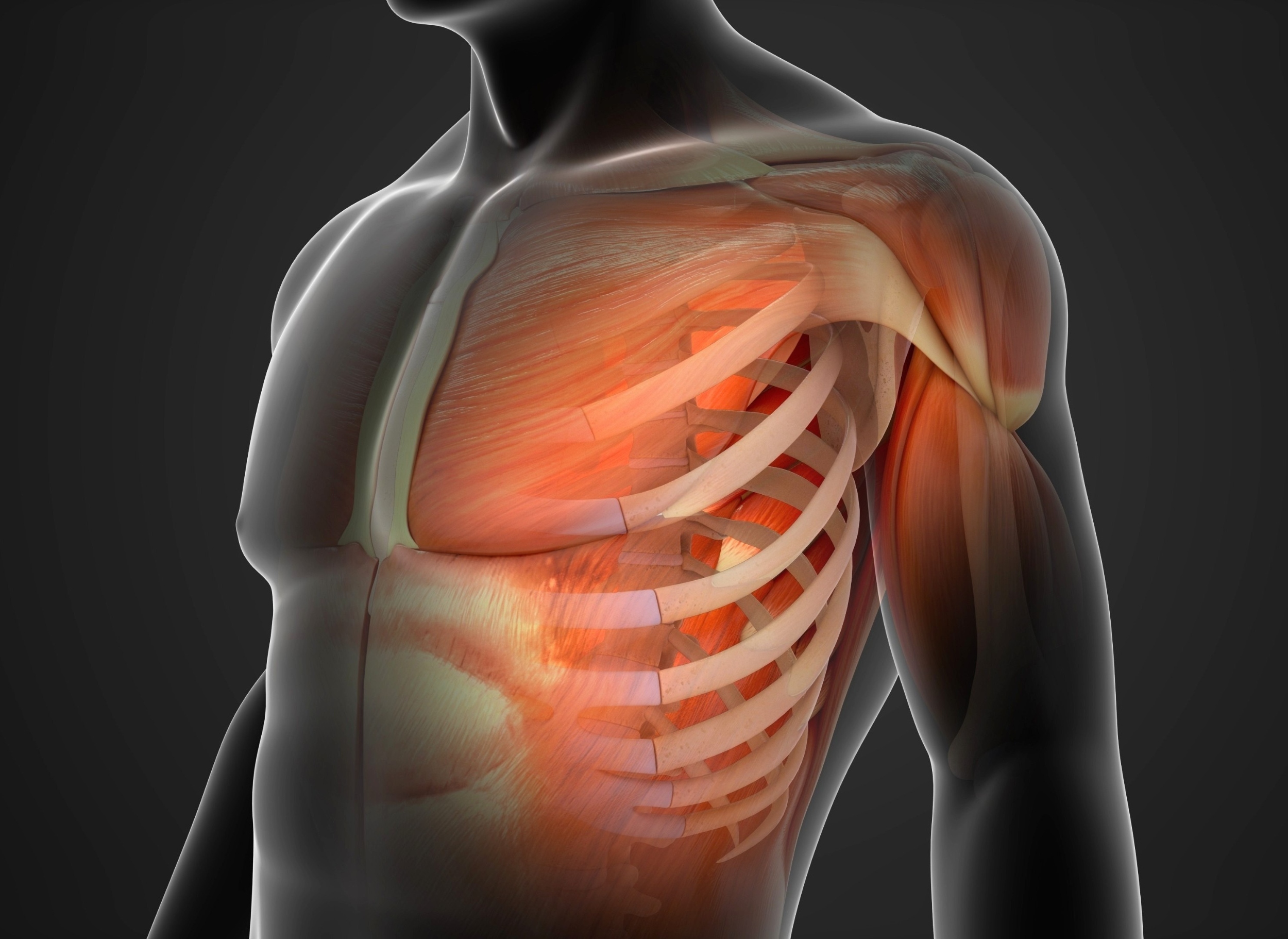
Skeletal muscle has been shown to store a molecular record of past inactivity, and new evidence reveals that youth and age encode that record in fundamentally different ways.
After a second enforced stretch of stillness, young muscle mounts a…

Five minutes alone will not create bodybuilder-level muscle. But it can build functional strength, especially for beginners.
Muscle adapts to stress. If someone who is sedentary begins doing daily squats and push-ups, even for five minutes, the…
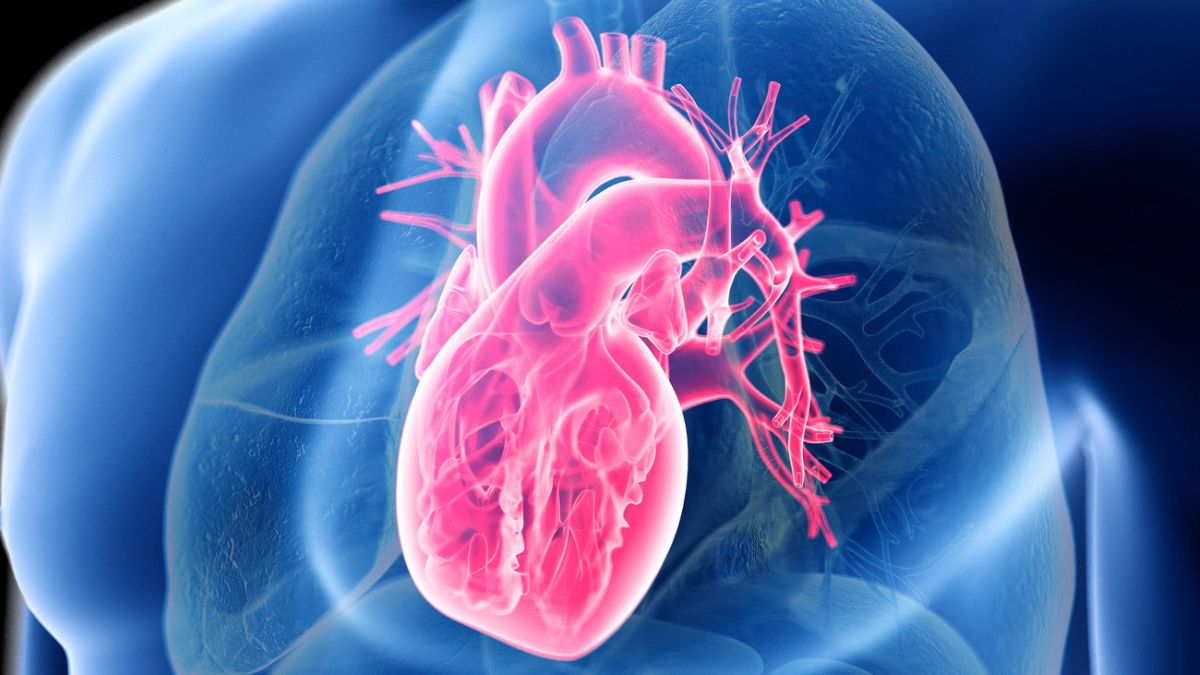
The key to heart health isn’t cutting down on pasta or potatoes, new evidence suggests; it’s not even a low-fat diet.
A study that tracked nearly 200,000 men and women in the US for around 30 years has now found that some low-fat and low-carb…
![[Campaign] UNAIDS: Zero Discrimination Day 2026](https://afnnews.qaasid.com/wp-content/uploads/2026/02/unaids.png)
Zero Discrimination Day will take place on 1 March 2026 with the theme: People first.
This year, UNAIDS shines a light on the persistent discrimination faced by people living with and at risk of HIV, discrimination that undermines…