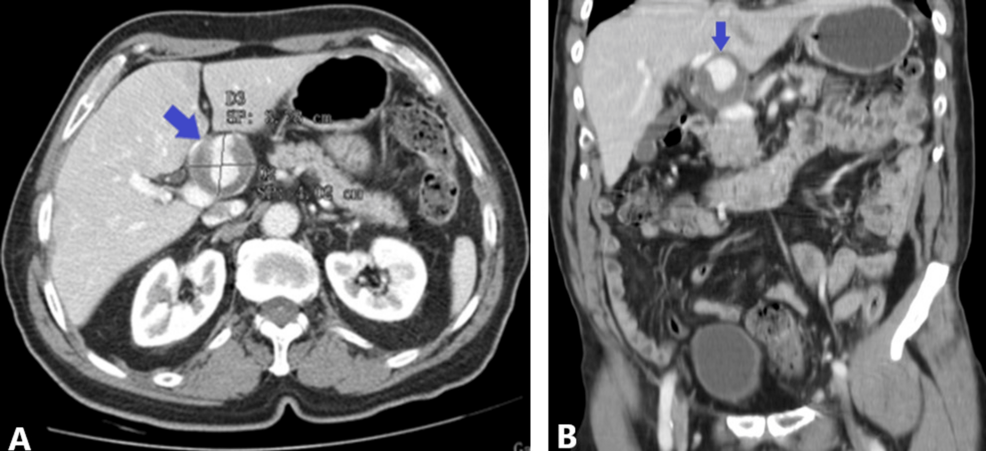
Category: 6. Health
-

ESMO Preceptorship on Hereditary Cancer Genetics 2026: Lugano
The ESMO Preceptorship on Hereditary Cancer Genetics 2026 is an intensive educational course scheduled for May 11–12, 2026, in Lugano, Switzerland.
Co-chaired by Judith Balmaña and Marjolijn Ligtenberg, this program is…
Continue Reading
-

ESMO Deep Dive: Genitourinary Cancers: Improving Outcomes in High-Risk Localized Prostate Cancer 2026
The ESMO Deep Dive: Genitourinary Cancers: Improving Outcomes in High-Risk Localized Prostate Cancer 2026 is a virtual scientific webinar scheduled for May 6, 2026.
This session focuses on the clinical management of…
Continue Reading
-

ESMO Guidelines: Real World Cases – Webinar Series: Bladder Cancer 2026
The ESMO Guidelines: Real World Cases – Webinar Series: Bladder Cancer 2026 is a specialized virtual scientific meeting scheduled for April 29, 2026, focused on translating the latest ESMO Clinical Practice Guidelines…
Continue Reading
-

Dupilumab Effective for Children with Severe Atopic Dermatitis in Real World Setting
Dupilumab’s efficacy and safety findings among children aged 6 months to 5 years with
severe atopic dermatitis are consistent with previous results observed in those with moderate-to-severe disease, new data suggest.1Audrey Lasek, MD, from…
Continue Reading
-

Cervical cancer vaccine
Sindh’s HPV vaccine push faces resistance rooted in deep-seated public mistrust, misinformation
Pakistan has always had a turbulent history with…
Continue Reading
-

Mohammad Sabry Alkady: When ‘Low-Dose, Long-Term’ Makes a Long-Lasting Difference
Mohammad Sabry Alkady/Facebook
Mohammad Sabry Alkady, Professor of Medical and Radiation Oncology at Faculty of Medicine at Ain Shams University, Egypt, shared a post on
Continue Reading
-
US sees spike in flu cases in December, after most severe season since 2018 | US news
The United States has seen the number of influenza cases climb significantly in December, coming after the most severe flu season since 2018, the Centers for Disease Control and Prevention said.
It’s not yet clear whether there will be an…
Continue Reading