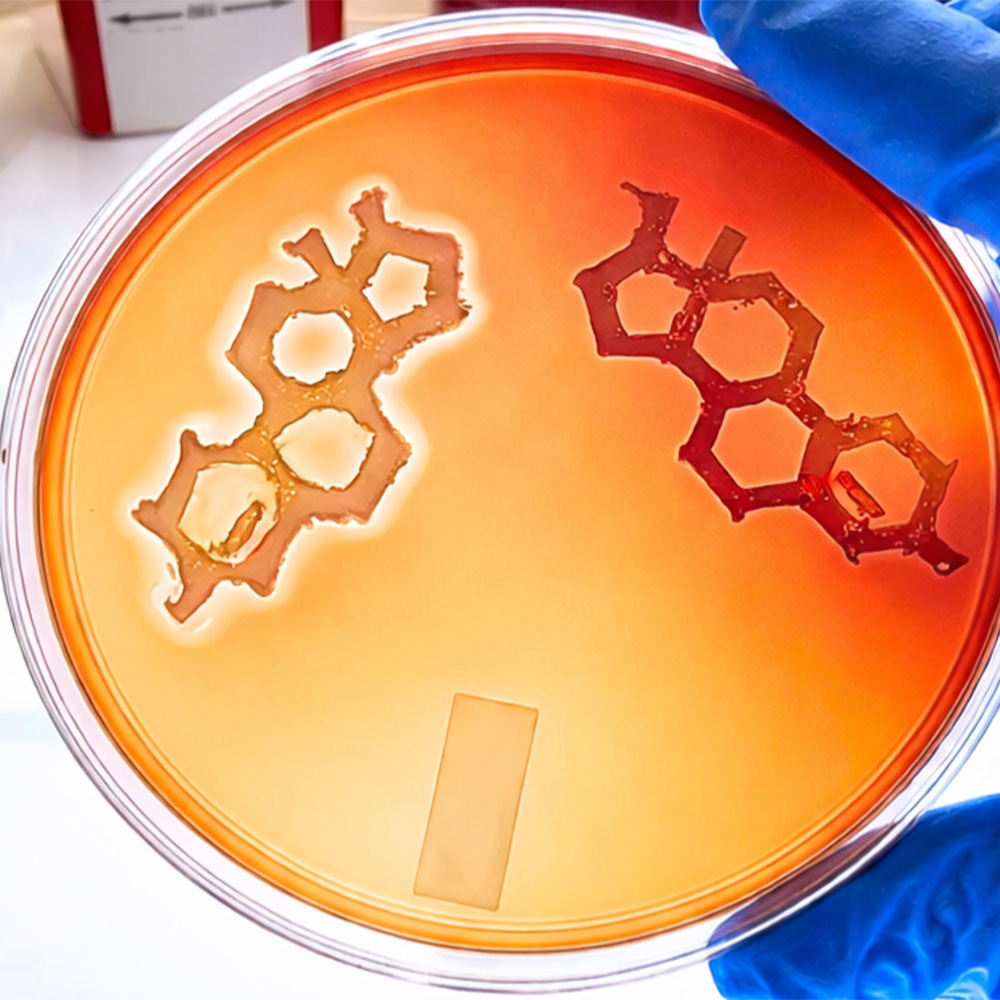
This laboratory image shows Staphylococcus aureus bacteria streaked in the shape of a sex steroid, like testosterone. The left shape is of wild-type S. aureus, with the lighter halo around the shape indicating hemolysis,…
Category: 6. Health
-

A New Antibody Treatment For Breast Cancer
A year of trastuzumab emtansine (T‑DM1) can keep about 98% of women with small, early‑stage HER2‑positive breast cancer alive and cancer‑free at three years, with far less nerve damage and hair loss than standard…
Continue Reading
-
New pathway found connecting liver congestion to fibrosis and cancer
Researchers from The University of Osaka find that chronic liver congestion is linked to severe liver diseases through a specific signaling pathway in liver sinusoidal endothelial cells – key cells lining the liver’s tiny blood…
Continue Reading
-
‘Nearly Every’ Child With Measles Suffers This Hidden Threat – Medscape
- ‘Nearly Every’ Child With Measles Suffers This Hidden Threat Medscape
- 7 reasons why measles is more dangerous than you think Gavi, the Vaccine Alliance
- Can contracting measles compromise your immunity to other diseases? The Colorado Sun
- Why…
Continue Reading
-
Recommendations for influenza vaccine composition for the 2026-2027 northern hemisphere season
The World Health Organization (WHO) today announced recommendations for the viral composition of influenza (or “flu”) vaccines for the 2026-2027 northern hemisphere influenza season. The announcement was made following a 4-day consultation…
Continue Reading
-

AI-Driven X-Ray Innovation Accelerates Early Arthritis Detection
Artificial intelligence is rapidly transforming healthcare, particularly in diagnostic imaging and predictive analytics. As digital health services expand worldwide, AI-driven tools are helping clinicians detect diseases earlier, improve patient…
Continue Reading
-

Zero-alcohol ads may increase teens’ intent to drink alcohol
Zero-alcohol ads may look harmless, but new research suggests they are associated with stronger brand loyalty and drinking intentions among teenagers, raising fresh questions about whether these campaigns indirectly promote alcohol…
Continue Reading
-
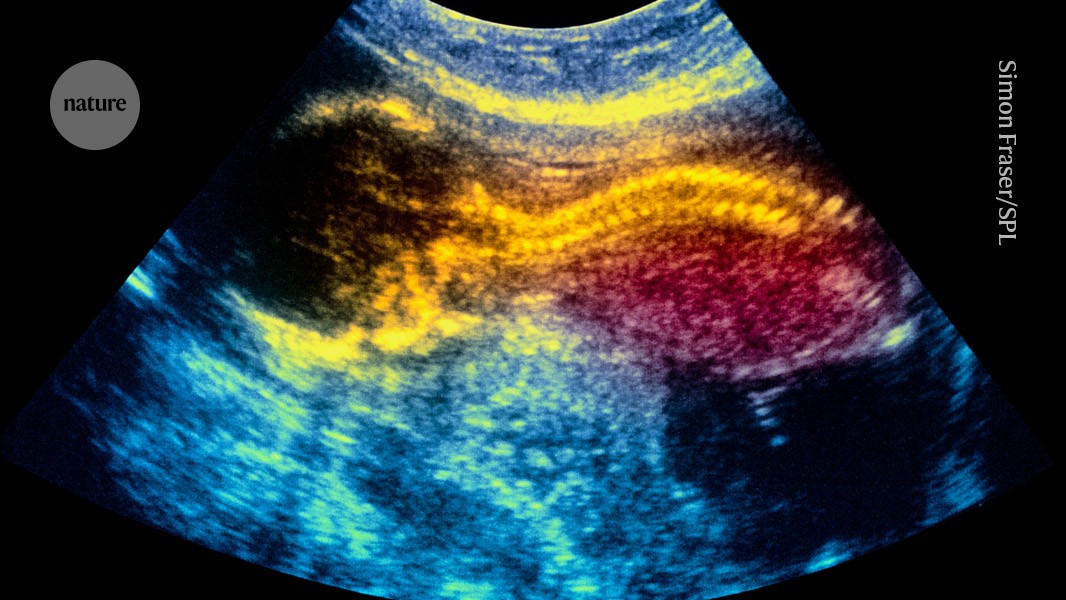
World-first stem-cell therapy shows promise for treating spina bifida in the womb
An in utero treatment using placenta-derived stem cells could treat infants with a neural-tube condition called spina bifida. Credit: Simon Fraser/Science Photo Library
Stem cells applied to the exposed spinal cords of fetuses in utero could…
Continue Reading
-

Nanoplastics Can Interact with Salmonella to Affect Food Safety, Study Shows
Newswise — URBANA, Ill. – Plastic products are ubiquitous in our food supply chain, shedding microplastics into every part of the human ecosystem. As they degrade, microplastics break down into even smaller fragments called…
Continue Reading