- Think 2025 Was a Big Year for Health News? Fasten Your Seat Belts Bloomberg.com
- A diplomat’s tears, 200 snake bites, drone pix: Goats and Soda’s top stories in 2025 NPR
- 6 Diseases to Watch Out for in 2026 BlackDoctor.org
- From Monkeypox to…
Category: 6. Health
-
Think 2025 Was a Big Year for Health News? Fasten Your Seat Belts – Bloomberg.com
-

Exercise won’t help you lose much fat. Changing this will
If you want to lose weight, exercise doesn’t really matter.
That doesn’t sound right, does it? After all, for decades we’ve been told that the way to burn off excess calories is simple: move more. Have a slice of cake? No problem, just make…
Continue Reading
-
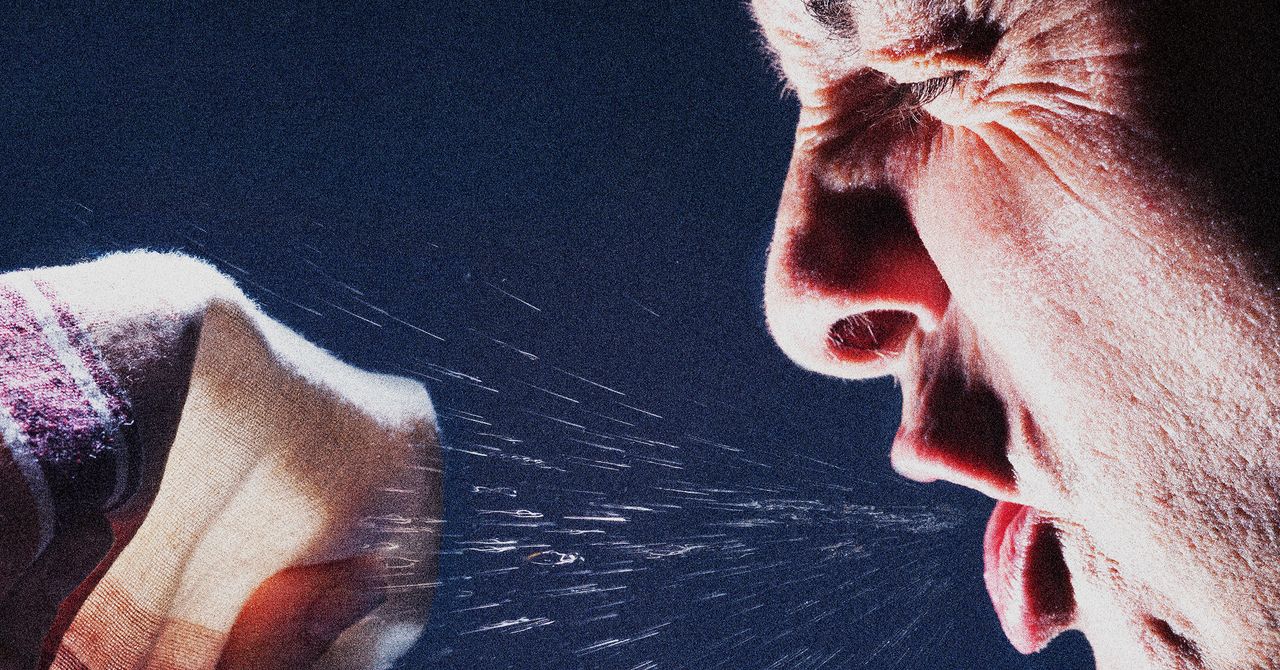
How to Make It Through Cold and Flu Season
You may not be able to prevent catching a cold or the flu, but you can greatly reduce your chances and decrease the likelihood that you’ll have a severe case if you do get sick. The well established advice meant to keep you healthy also protects…
Continue Reading
-
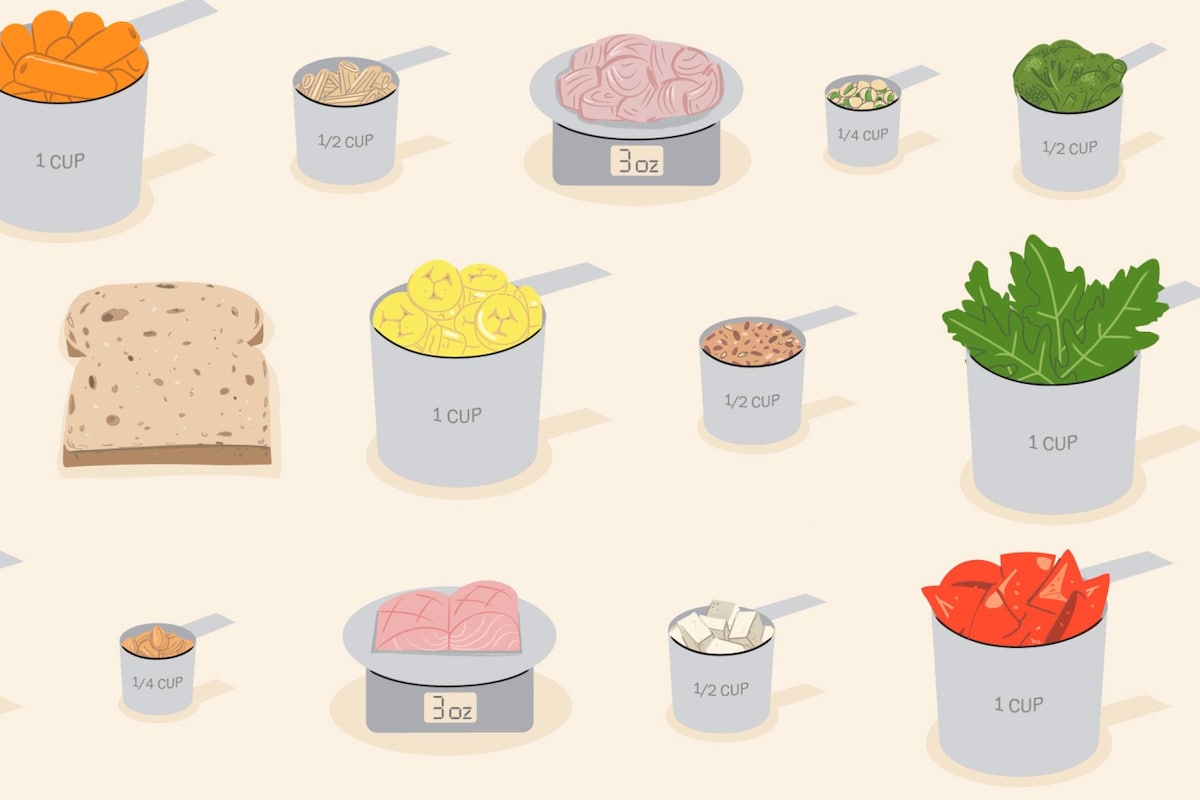
Are you eating for longevity? Take this quiz to find out.
Eating healthy food can add years to your life. It can lower your odds of developing heart disease, cancer, dementia and other chronic diseases.
But how do…
Continue Reading
-
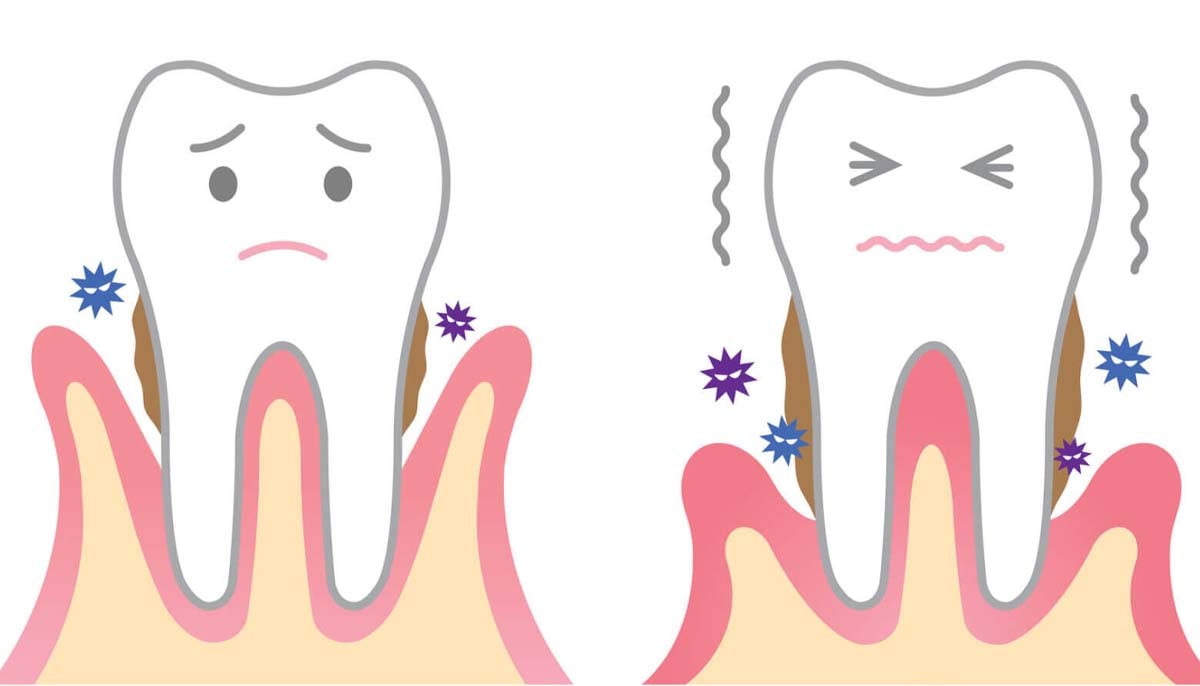
Mouth bacteria may raise risk of serious brain problems
A new study from researchers in Japan has found a possible link between gum disease and…
Continue Reading
-

Anticipatory Nursing Improves COPD Quality of Life – European Medical Journal Anticipatory Nursing Benefits in COPD Care
IN CHRONIC obstructive pulmonary disease, anticipatory nursing, boosted QoL, eased distress, and reduced complications in 6 months.
Anticipatory Nursing in Chronic Obstructive Pulmonary Disease
Psychological distress and persistent…
Continue Reading
-
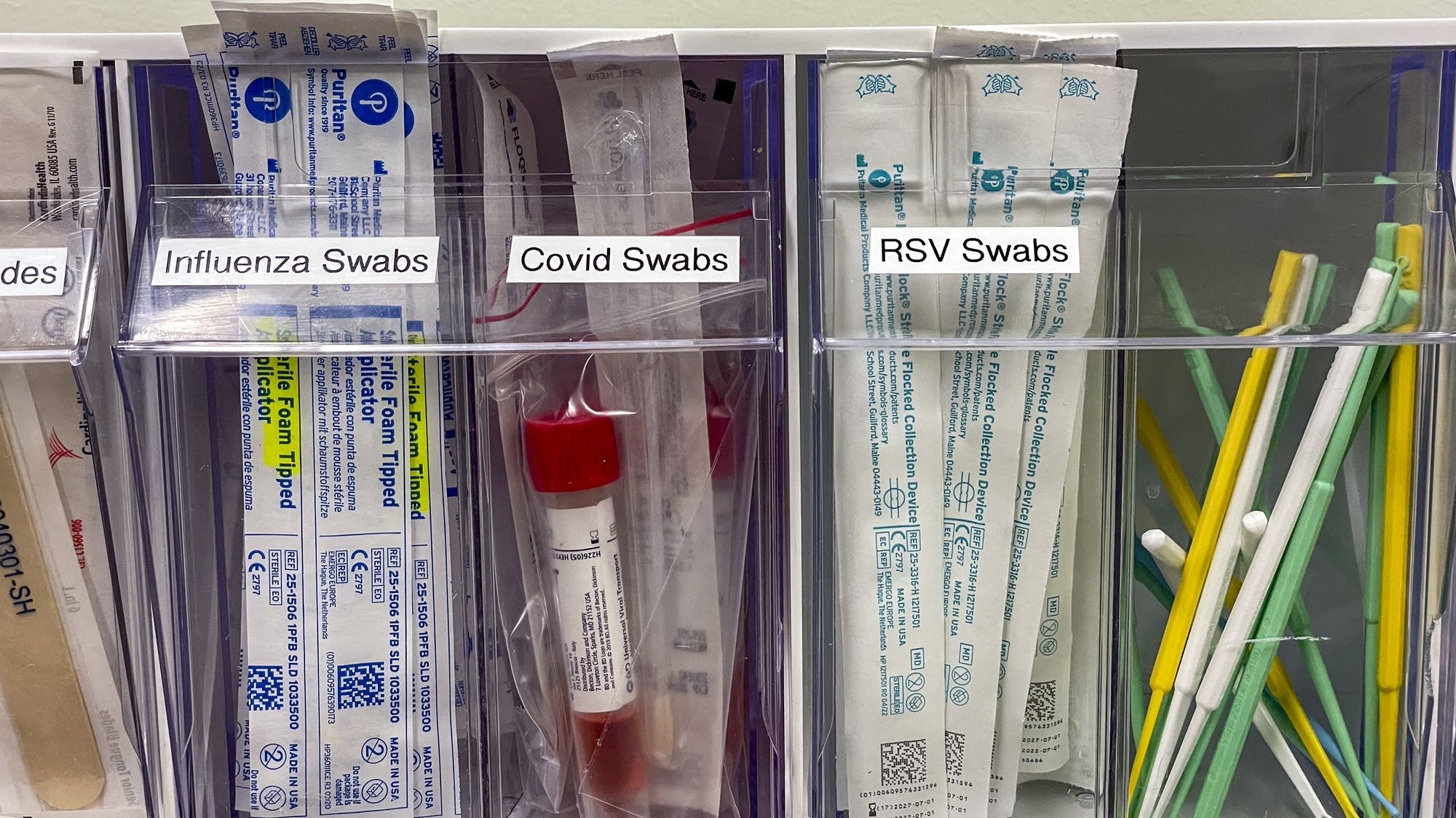
Flu positivity rates and hospitalizations increase in Minnesota – MPR News
- Flu positivity rates and hospitalizations increase in Minnesota MPR News
- Health Department discusses importance of immunizations somerset-kentucky.com
- Respiratory illnesses lead to more than 180 hospitalizations in past week News 12 -…
Continue Reading
-

Frailty, depression in older adults may together account for 17 % of dementia risk: Study
Frail participants were more likely to be female, have higher body weight, live with multiple long-term conditions, and lower educational attainment. They were also 2.5 times more likely to be diagnosed with dementia |Image…
Continue Reading
-
Jersey flu cases slow but islanders warned to stay alert
Ill people have been urged to stay at home to slow the spread of flu and GPs urged people to get vaccinated.
A spokesperson for Public Health Jersey said anecdotal evidence suggested there had been a slight increase in islanders getting the…
Continue Reading
