Dr Orange, a senior lecturer in clinical exercise physiology, said it showed “even a single workout” could make a difference.
“What’s remarkable is that exercise doesn’t just benefit healthy tissues, it sends powerful signals through the…

Dr Orange, a senior lecturer in clinical exercise physiology, said it showed “even a single workout” could make a difference.
“What’s remarkable is that exercise doesn’t just benefit healthy tissues, it sends powerful signals through the…
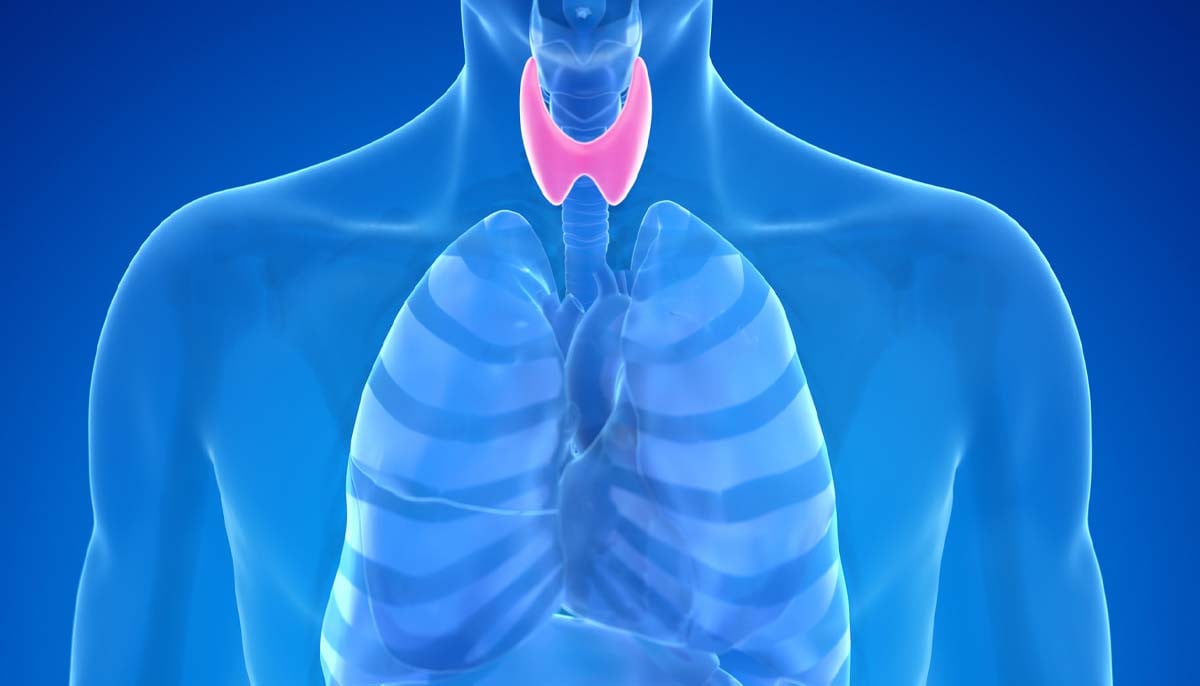
The NHS has issued a warning to anyone taking levothyroxine to treat their…
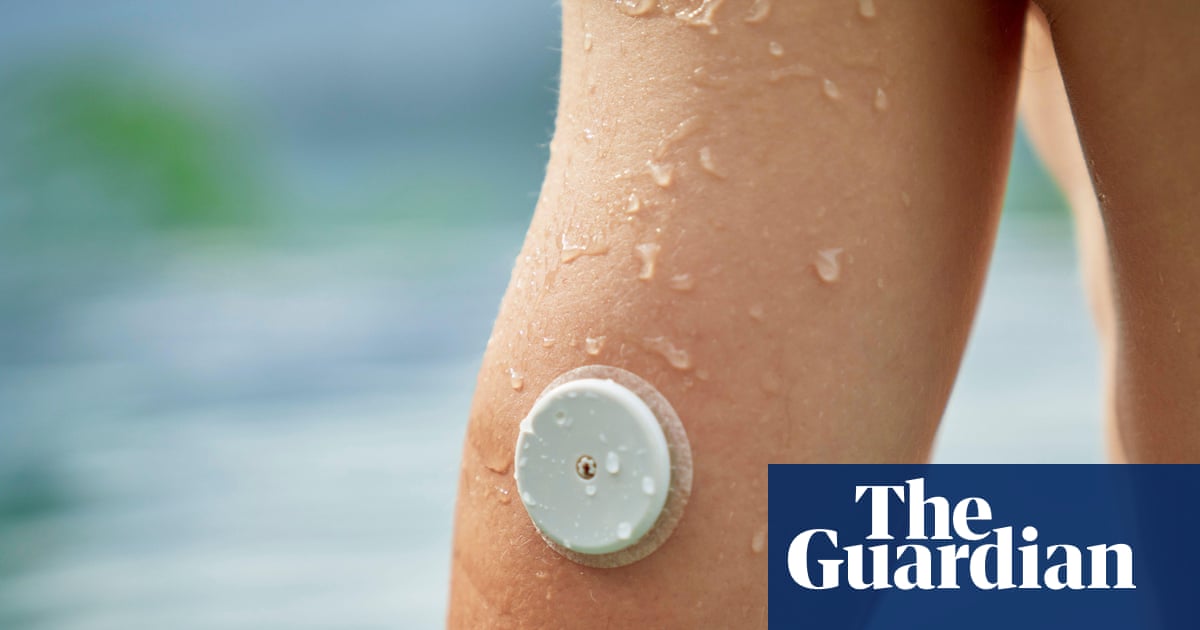
People from ethnic minority backgrounds in England are less likely to have access to the latest diabetes technology, despite being more likely to live with the condition, according to analysis.
Devices such as a continuous glucose monitor (CGM)…

It was a Monday evening in mid-December and a woman was in labour in the Rotunda Hospital when the healthcare facility learnt there were no neonatal beds available in Dublin.
Consequently, the woman was transferred via ambulance to deliver her…

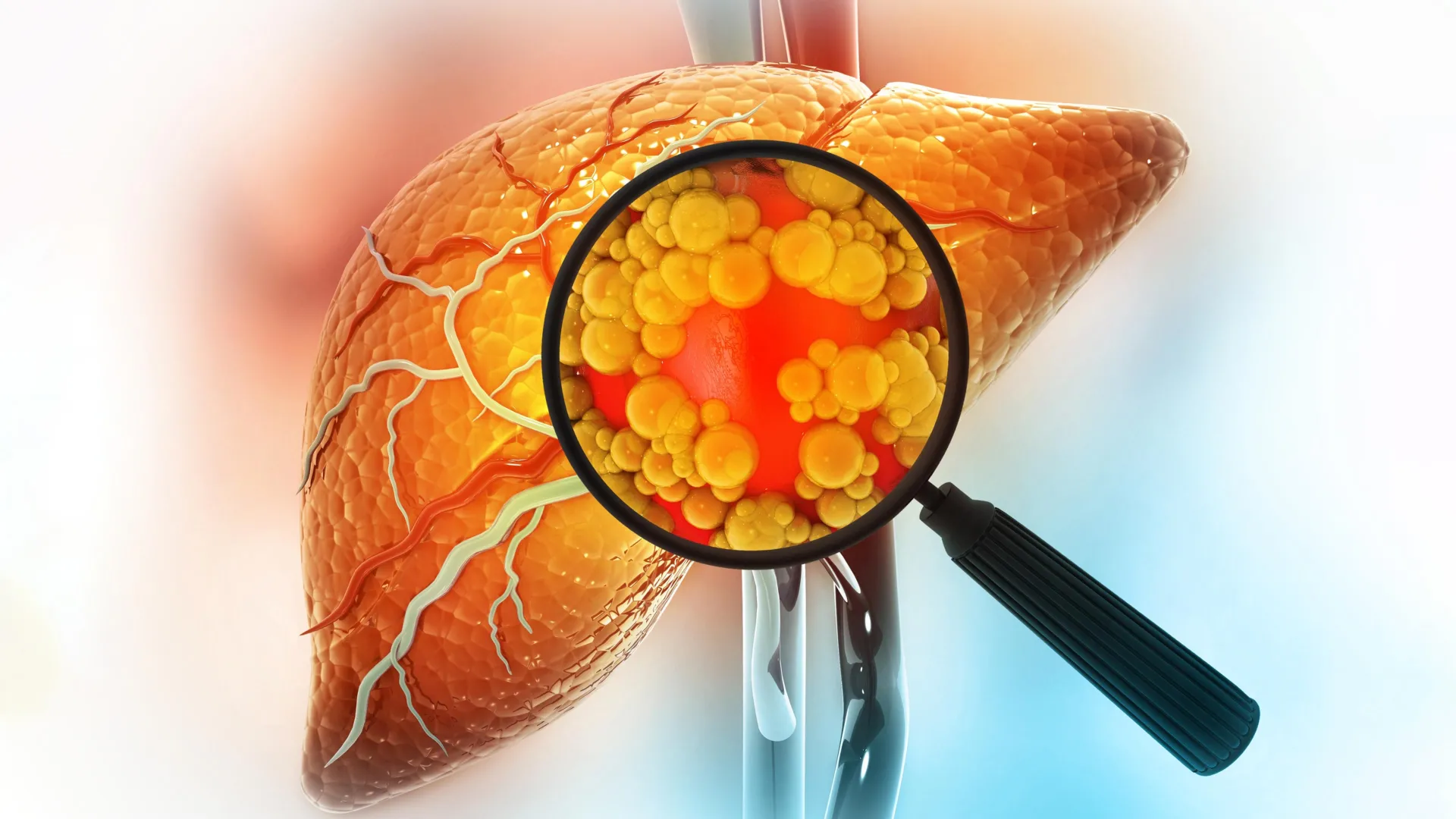
A diet high in fat is one of the strongest contributors to liver cancer risk. New research from MIT sheds light on why this happens, showing that fatty diets can fundamentally alter liver cells in ways that make cancer more likely to develop.
The…
Health security faces ongoing challenges from infectious diseases, requiring innovative solutions and collaboration. The Nipah virus, transmitted by flying foxes (fruit bats), is a persistent…
An unexpected organism, the Drosophila fruit fly, is proving to be a powerful model organism for studying human diseases and developing potential treatments, including protection against…

The dawn of a new year brings visions of an idealised version of yourself. Fresh-faced, we eagerly pile our to-do lists with things we’ve been putting off and ambitions to aim for. But the energy that comes after a few days off quickly…

BOCA RATON, FL – January 1, 2026 – Marcos A. Nores, M.D., board-certified cardiac surgeon, has joined Baptist Health Heart & Vascular Care as medical director of Christine E. Lynn Heart & Vascular Institute at…