Catherine Cook sold her business to pay out of pocket for Keytruda for her aggressive breast cancer treatment.
Photo: Supplied
An Auckland woman who is self-funding treatment for her rare, aggressive breast cancer is fed up after…

Catherine Cook sold her business to pay out of pocket for Keytruda for her aggressive breast cancer treatment.
Photo: Supplied
An Auckland woman who is self-funding treatment for her rare, aggressive breast cancer is fed up after…
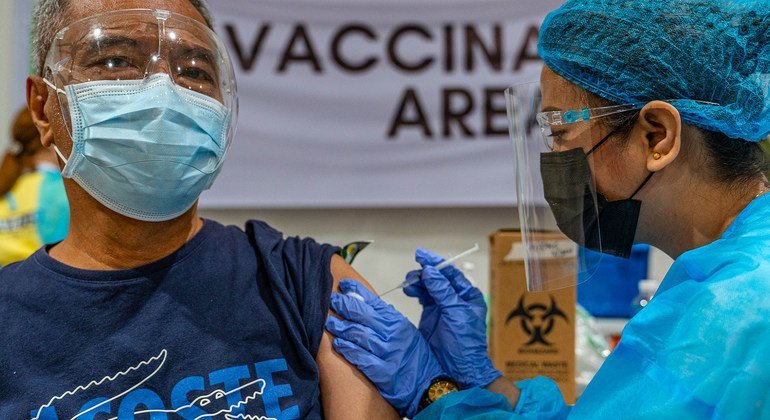
Although COVID-19 no longer causes the widespread disruption seen during the global health emergency, the virus continues to hospitalize and kill people across Europe and neighbouring regions.
Studies led by the WHO Regional Office for Europe…
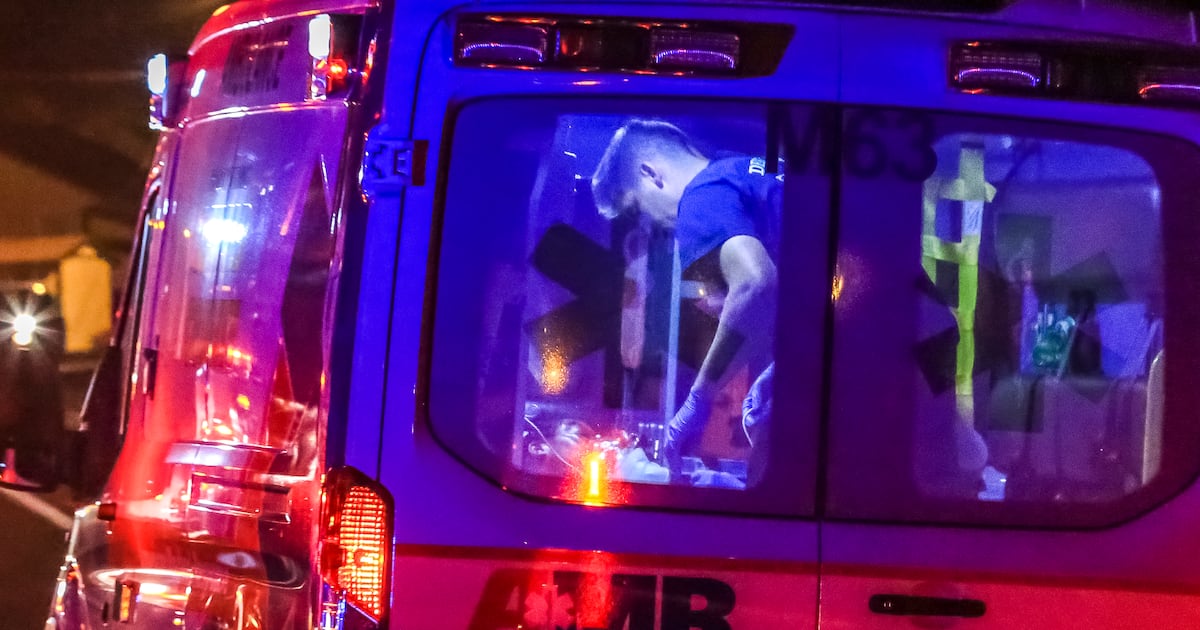
The 2025-2026 flu season is likely going to get worse, officials say.
American Medical Response said 911 calls related to influenza have increased 60% within the county over the past several weeks. (AJC file)
DeKalb County issued an alert…
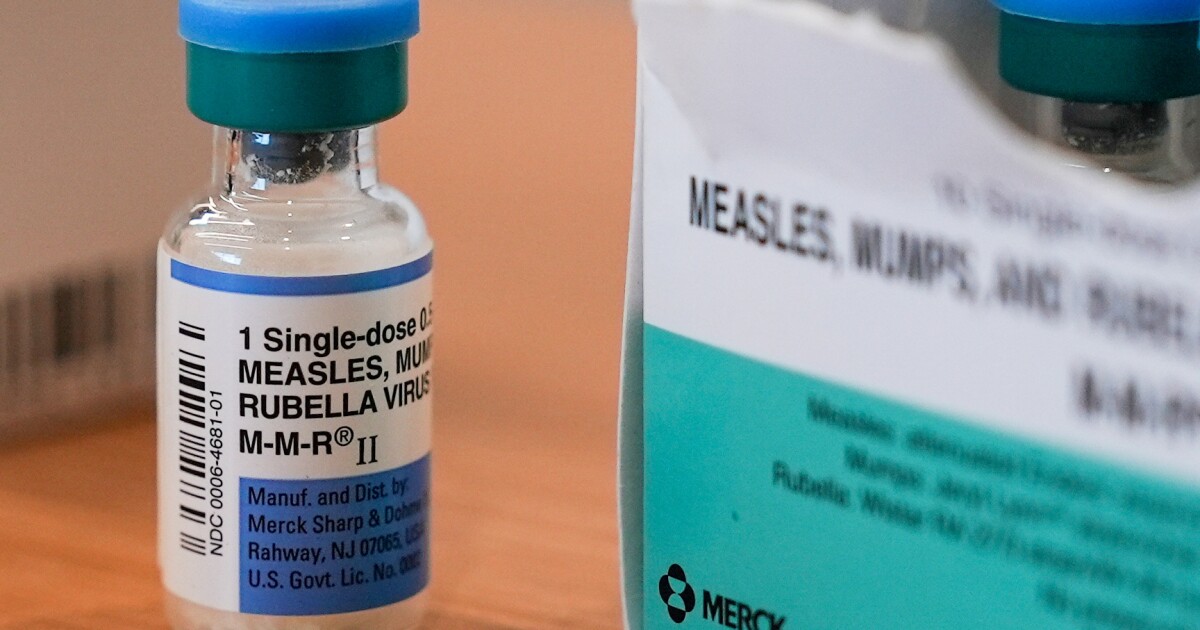
The North Carolina Department of Health and Human Services announced a case of measles on Wednesday in a child in Polk County. It’s the second case in the state this year and the first related to an…

At The University of New Mexico’s Health Sciences Center (HSC), 2025 was a year of groundbreaking research.
Making up the bulk of the top 10 most-viewed stories on the HSC Newsroom were articles detailing the efforts of the HSC’s bold…

Diligent public health efforts nearly eliminated syphilis 20 years ago.
Stopping outbreaks involved extensive testing and treatments for the sexually transmitted infection (STI) in affected people, as well as tracing, testing, and treating their…

A randomized clinical trial led by Johns Hopkins researchers finds that giving patients a next-day HIV viral load (VL) test result didn’t improve rates of care-seeking for antiretroviral therapy (ART) or HIV preexposure prophylaxis…

A randomized clinical trial led by Johns Hopkins researchers finds that giving patients a next-day HIV viral load (VL) test result didn’t improve rates of care-seeking for antiretroviral therapy (ART) or HIV preexposure prophylaxis…