Vegetarians have a substantially lower risk of five types of cancer, a landmark study on the role of diet has revealed.
The research, using data from more than 1.8 million people who were tracked over many years, found that vegetarians had a 21%…

Vegetarians have a substantially lower risk of five types of cancer, a landmark study on the role of diet has revealed.
The research, using data from more than 1.8 million people who were tracked over many years, found that vegetarians had a 21%…
A multiomic atlas of the aging human hippocampus uncovers how epigenetic regulation of neural stem cells and immature neurons may shape cognitive decline or resilience in later life.
Study: Human hippocampal neurogenesis in…
It’s widely known that breastfeeding impacts the health of both mother and child, but the underlying biology that leads to these effects has been understudied. In a review article publishing in the Cell Press journal Trends…

Messenger RNA (mRNA) technology is transforming medicine by providing our cells with genetic instructions to produce proteins that help the immune system prevent or fight a wide range of diseases, including cancer and other rare…

A study published today in the journal Science reveals how jumping fragments of human DNA, a type of genetic parasite, destabilise the cancer genome. Unstable genomes are a fertile playground for cancer evolution, giving malignant…

A researcher at the University of Missouri has made a promising breakthrough in the quest to help people with amyotrophic lateral sclerosis (ALS), the neurodegenerative disorder commonly known as Lou Gehrig’s disease.
In a recent…

Disclaimer: Early release articles are not considered as final versions. Any changes will be reflected in the online version in the month the article is officially released.
Author affiliation: US Centers for Disease Control and…
A review has found that parents are increasingly refusing vitamin K injections for their newborns, an intervention that lowers the risk of life-threatening brain bleeds in babies. Babies have naturally low levels of vitamin K, making this…
A new non–peer-reviewed study estimates that measles outbreaks in the United States cost more than $244 million in 2025 alone and warns that even modest declines in childhood measles, mumps, and rubella (MMR) vaccination could trigger billions…
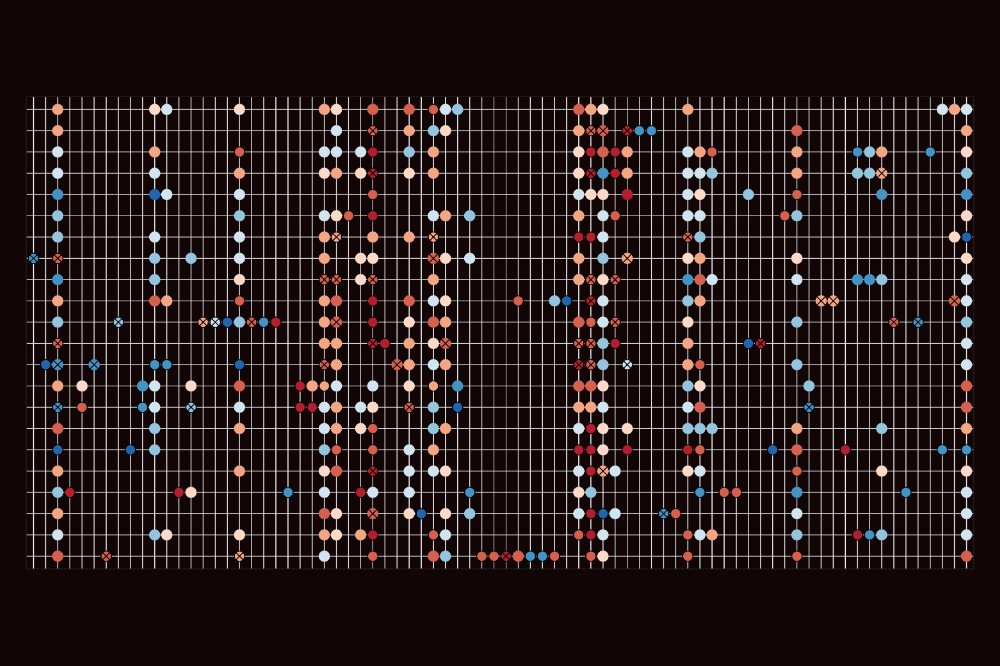
Dot plot showing the aging-vulnerable cell types across the entire body. (Cao lab)
As we age with each passing year, we become more susceptible to chronic diseases like cancer, heart disease, and dementia. Scientists have long focused on…