New research from Sylvester Comprehensive Cancer Center at the University of Miami Miller School of Medicine suggests that women who live near federally designated Superfund sites face a higher risk of developing aggressive forms of breast cancer…
Category: 6. Health
-
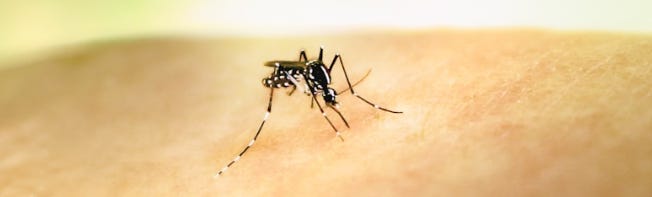
Global chikungunya 2025 – by Robert Herriman
According to a World Health Organization (WHO) Rapid Risk Assessment (RRA) published this week, from 1 January to 10 December 2025, 502,264 CHIKV disease cases, including 208,335 confirmed cases, and 186 CHIKV deaths, were reported globally.
Continue Reading
-

Case definitions for severe acute respiratory infections may underestimate true incidence
A systematic review and meta-analysis finds that the definition of severe acute respiratory infection (SARI) used by the World Health Organization (WHO) demonstrated reduced sensitivity and low specificity in children, suggesting that…
Continue Reading
-

Top 5 Most-Read Cardiovascular Content of 2025
The top cardiovascular managed care news of 2025 covered the full range of care, from research to drug approvals to patient monitoring.
Among this year’s top content, a pair of studies published in The American Journal of Managed Care® (AJMC
Continue Reading
-

Flu is on a major upswing in the U.S., new CDC data shows : Shots
There have been at least 7,500,00 illnesses and 3,100 deaths from flu this season, according to CDC data. And flu cases are…
Continue Reading
-
A Deadly, Drug-Resistant Fungus Threatens People Around the World, Scientists Warn
A deadly, drug-resistant fungus is extending its tendrils around the world. Research out this month finds that the public health threat of Candida auris has steadily climbed over time.
Researchers reviewed the scientific…
Continue Reading
-

Infant screen time linked to anxiety, slower cognition • The Register
If you’re thinking of plopping your infant in front of a screen to get some peace and quiet, you might want to reconsider – higher screen exposure in infancy was linked to longer decision times later on and higher anxiety symptoms in the teenage…
Continue Reading
-
RSV confers similar risk of poor outcomes in hospitalized patients as flu over time, data suggest
Relative to uninfected control patients, those hospitalized with acute respiratory infections (ARIs) due to respiratory syncytial virus (RSV) or influenza were at substantially higher adjusted risk for all-cause death, heart attack, exacerbation…
Continue Reading
-

More than half a million chikungunya cases reported globally in 2025
CDC / James Gathany Through December 10, the world has seen more than 500,000 chikungunya cases worldwide, with almost 300,000 in the Americas region alone, the World Health Organization (WHO) reported in a risk assessment yesterday.
With a…
Continue Reading
-
In a year of steep challenges, there were still shining moments in global health – NPR
- In a year of steep challenges, there were still shining moments in global health NPR
- Stronger together – milestones that mattered in 2025 World Health Organization (WHO)
- UN: 2025 Marked by First Agreement on Pandemics radioangulo.cu
- WHO chief…
Continue Reading
