Introduction
Refractive surgery has seen a steady rise in popularity since its introduction over 25 years ago.1–3 Myopia accounts for 80% of refractive diagnoses among patients undergoing Laser Vision Correction, followed by hyperopia at 15%.4…

Refractive surgery has seen a steady rise in popularity since its introduction over 25 years ago.1–3 Myopia accounts for 80% of refractive diagnoses among patients undergoing Laser Vision Correction, followed by hyperopia at 15%.4…
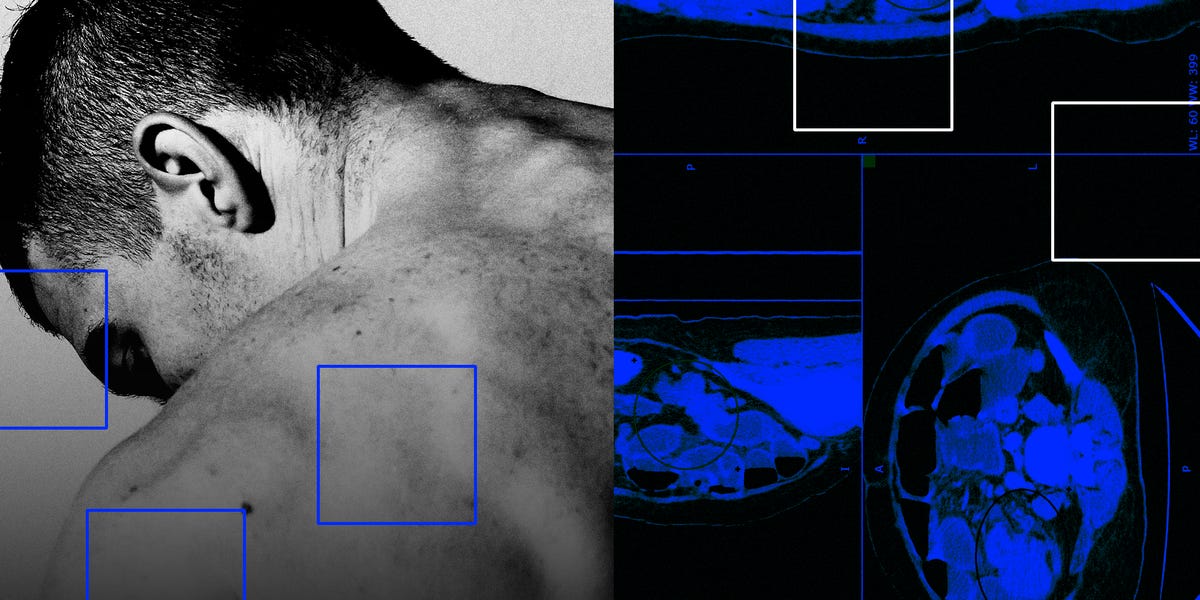
Five years ago, Tim Cannon, a cancer doctor in Virginia, saw that his colon cancer patients were getting younger and their cancer was more aggressive. He had just diagnosed three people in their 30s with late-stage colon…
ULAN BATOR, Dec. 30 (Xinhua) — Mongolia’s measles death toll has reached 12 after one more related death was registered, the National Center for Communicable Diseases (NCCD) said on Tuesday.
Meanwhile, 22 people remained in hospital,…
ULAN BATOR, Dec. 30 (Xinhua) — Mongolia’s measles death toll has reached 12 after one more related death was registered, the National Center for Communicable Diseases (NCCD) said on Tuesday.
Meanwhile, 22 people remained in hospital,…

Gestational diabetes rose every single year in the U.S. from 2016 through 2024, according to a new Northwestern Medicine analysis of more than 12 million U.S. births. The condition, which raises health risks for both mother and…


Immune cells called B cells make antibodies that fight off invading bacteria, viruses and other foreign substances. During their preparation for this battle, B cells transiently revert to a more flexible, or plastic, stem-cell-like…