The nervous system does an astonishing job of tracking sensory information, and does so using signals that would drive many computer scientists insane: a noisy stream of activity spikes…
Category: 6. Health
-

Projections of Future COPD Economic Impact “Unsustainable”
The global direct costs attributable to COPD are projected to exceed $24 trillion by 2050, according to a recent forecast published in Chest.
“The current economic burden attributable to COPD is substantial. For instance, in the United States, a…
Continue Reading
-
Opinion: The miracle of curing Alzheimer’s? No longer an impossibility. – MarketWatch
- Opinion: The miracle of curing Alzheimer’s? No longer an impossibility. MarketWatch
- Alzheimer’s Fully Reversed in Mice, Scientists Say Futurism
- Scientists reverse Alzheimer’s in mice and restore memory: Study ET HealthWorld
- New study shows…
Continue Reading
-

‘Long COVID and Workplace Safety’: New report from NSC
Washington — Employers can help reduce the safety risks of long COVID in the workplace through a multifaceted approach that focuses on the physical, cognitive and mental health challenges that affected employees face, according to a recent…
Continue Reading
-
Candida auris spreads globally as drug resistance and virulence increase, review finds – Medical Xpress
- Candida auris spreads globally as drug resistance and virulence increase, review finds Medical Xpress
- New findings on Candida auris open up potential targets for future therapies MedUni Wien
- Scientists find a weak spot in deadly fungus that shut…
Continue Reading
-
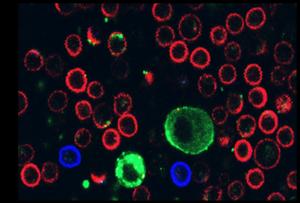
L-cystine alleviates necrotizing enterocolitis by regulating ferroptosis and Th17 cell differentiation via the IL-6/STAT3 pathway
Roberts, A. G., Younge, N. & Greenberg, R. G. Neonatal Necrotizing Enterocolitis: An Update on Pathophysiology, Treatment, and Prevention. Paediatr. Drugs 26, 259–275 (2024).
Alsaied, A.,…
Continue Reading
-
OHSU researchers find breast cancer drug boosts leukemia treatment
A research team at Oregon Health & Science University has discovered a promising new drug combination that may help people with acute myeloid leukemia overcome resistance to one of the most common…
Continue Reading
-
Rethinking Endpoints in Chronic Hepatitis B Treatment
IN A NEW expert analysis, hepatology researchers are calling for a strategic shift in how clinical trials and clinicians define successful treatment for chronic hepatitis B (CHB). While complete loss of hepatitis B surface antigen (HBsAg),…
Continue Reading
-
FLEX Study Data Highlight Role of Genomic Testing in Guiding Early-Stage Breast Cancer Treatment
Pharmacy Times: The FLEX study provides a depth of real-world genomic data rarely seen in oncology. How might this expanding dataset help pharmacists anticipate toxicity risks, optimize supportive-care strategies, or better counsel patients…
Continue Reading