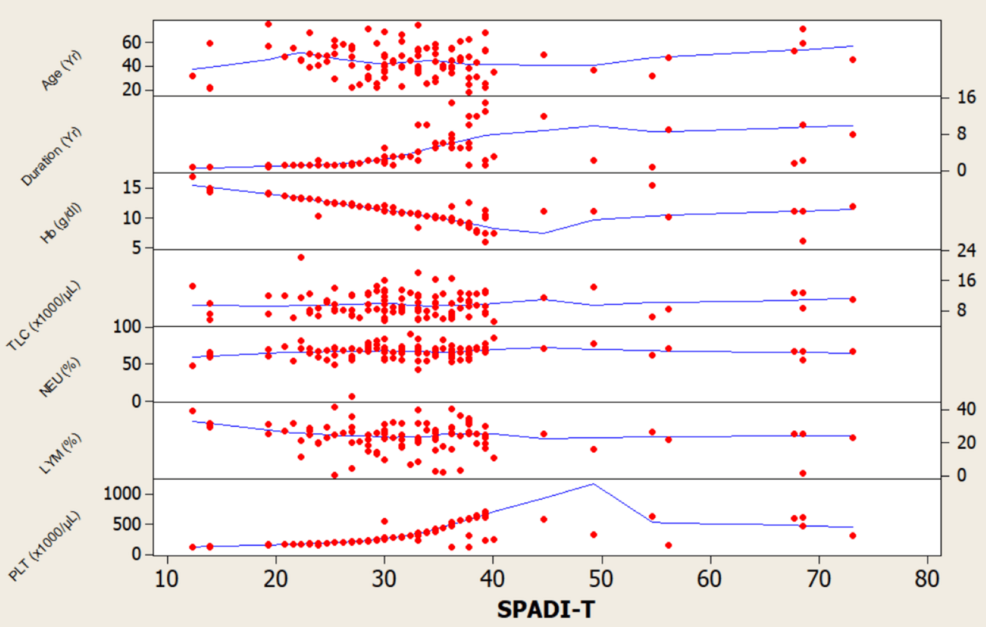
- DNA Methylation Patterns Linked to Gestational Diabetes Identified Across Pregnancy Stages geneonline.com
- New Clues Reveal How Gestational Diabetes Affects Offspring Eurasia Review
- Early Screening For Gestational Diabetes: Why Timely Detection…
Category: 6. Health
-
DNA Methylation Patterns Linked to Gestational Diabetes Identified Across Pregnancy Stages – geneonline.com
-

The Best Evening Habit to Support Digestion
- Unwind in the evening with a warm cup of herbal tea to support healthy digestion.
- Herbal teas like peppermint, ginger and fennel may ease discomfort and constipation risk.
- Eating fiber-rich foods, managing stress and staying active are also…
Continue Reading
-
Newark Airport passenger may have exposed others to measles, New Jersey Health Department says
NEWARK, New Jersey (WABC) — A passenger at Newark Airport may have exposed people to measles.
New Jersey’s Health Department says someone with the disease passed through the airport on Friday, December 19.
The person was in terminals B and C…
Continue Reading
-

Hands-on memories spark connection for dementia residents at western NSW aged care home
Creating meaningful, sensory activities for people living with dementia can be challenging, particularly when many traditional options feel more suited to children than adults with a lifetime of lived experience behind them.
At Cooinda Aged Care…
Continue Reading
-
Associations between peripheral neuropathy and cardiovascular complications in patients with type 2 diabetes mellitus: a cross-sectional study
Liu, Q. et al. Predicting the risk of incident type 2 diabetes mellitus in Chinese elderly using machine learning techniques. J. Personalized Med. 12 (6), 905 (2022).
Saeedi, P. et al….
Continue Reading
-

I was bitten by a tick — now I can’t eat beef or dairy – The Times
- I was bitten by a tick — now I can’t eat beef or dairy The Times
- US women issue warning after contracting shocking illness: ‘I felt like I was allergic to existing’ The Cool Down
- Tick-borne red meat allergy sees increased presence Madison…
Continue Reading
-

Australia issues health alert on fake rabies vaccine, Abhayrab®, in India
The Australian Technical Advisory Group on Immunisation (ATAGI) and Victoria’s Department of Health issued a health alert recently on the circulation of counterfeit batches of the rabies vaccine Abhayrab® in India since 1 November 2023.
People…
Continue Reading
-
Overlooked microbes may be essential for human health
Viruses and bacteria usually show up in headlines as villains. Yet a growing wave of research says that’s only half the story.
A team led by Flinders University microbial ecologist Jake Robinson argues it’s time to spotlight the upside,…
Continue Reading