- Colorectal cancer is rising in younger adults. Here’s who is most at risk and symptoms to watch for SFGATE
- Colorectal cancer: Symptoms and what causes it LiveNOW from FOX
- James Van Der Beek said there wasn’t a ‘red flag’ of his colorectal cancer…
Category: 6. Health
-
Colorectal cancer is rising in younger adults. Here's who is most at risk and symptoms to watch for – SFGATE
-

The Health Benefits of Black Sesame Seeds
Key Takeaways
- Black sesame seeds are a delicious and nutrient-packed way to elevate your meals and boost your health.
- These seeds are rich in antioxidants, healthy fats, and fiber, supporting heart, bone, and digestive health.
- With their bold…
Continue Reading
-

Q&A: Guiding Breast Cancer Treatment With Genomic Risk Stratification
In this Q&A with Lee Schwartzberg, MD, FACP, he discusses how genomic testing with MammaPrint and BluePrint guides treatment decisions for patients with HR-positive, HER2-negative breast cancer. He explains how risk stratification helps…
Continue Reading
-
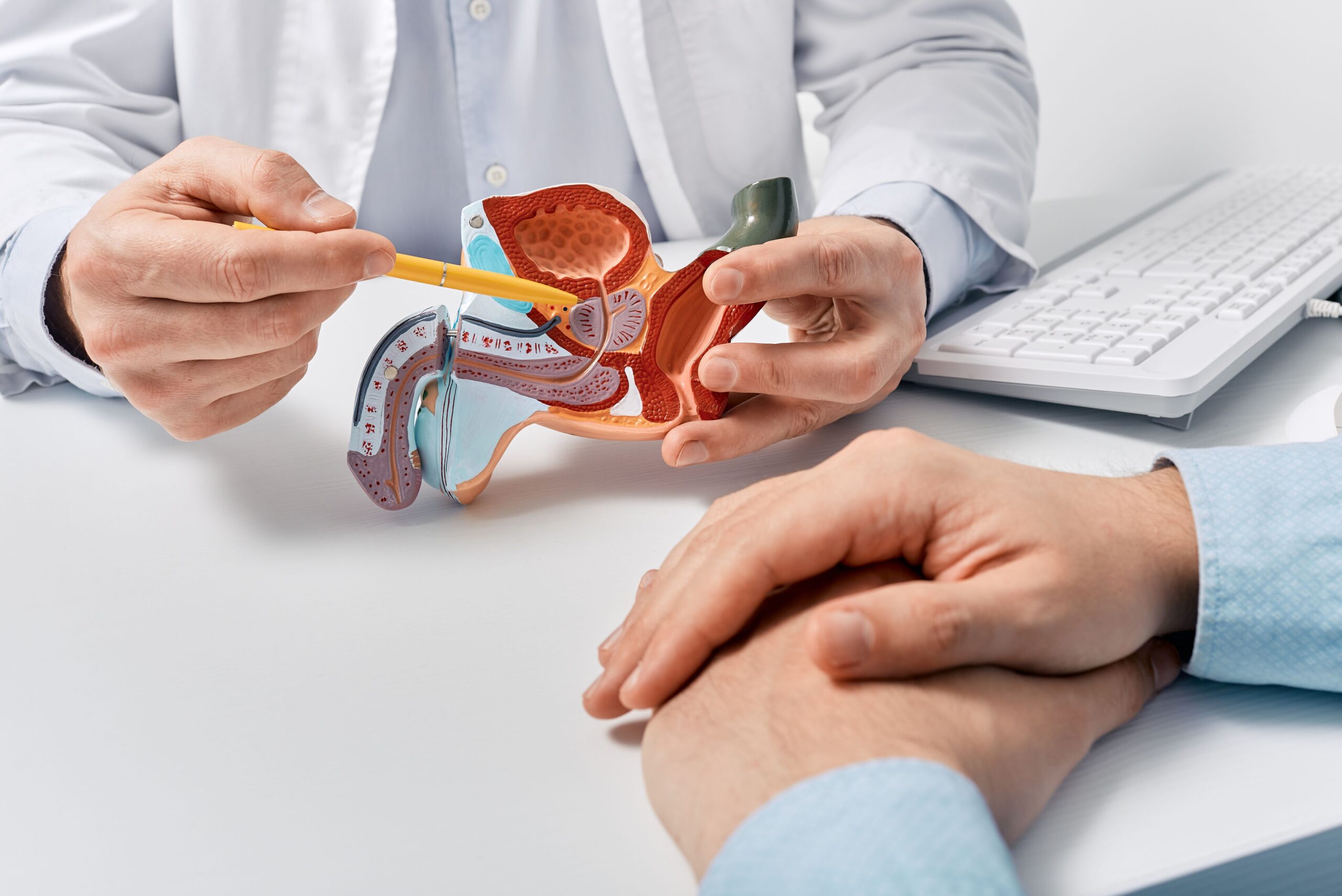
Growth Differentiation Factor-15: A Multifaceted Player in Prostate Cancer Biology and Therapeutic Targeting
Prostate cancer remains one of the most commonly diagnosed malignancies among men worldwide and continues to represent a significant cause of cancer-related morbidity and mortality. Despite advances in early detection and treatment, advanced and…
Continue Reading
-
Scientists Discover a Brain Circuit That Enhances Physical Endurance In Mice : ScienceAlert
The effects of exercise would not be nearly as powerful without the input of the brain, according to new research.
A study on mice has found a critical signal in the central nervous system that helps build physical endurance in the wider body…
Continue Reading
-
This New Blood Test Can Detect Cancer Before Tumors Appear – SciTechDaily
- This New Blood Test Can Detect Cancer Before Tumors Appear SciTechDaily
- Light-based sensor detects early molecular signs of cancer in the blood Medical Xpress
- CU Anschutz to participate in early cancer detection trial 9News
- Ochsner researching…
Continue Reading
-
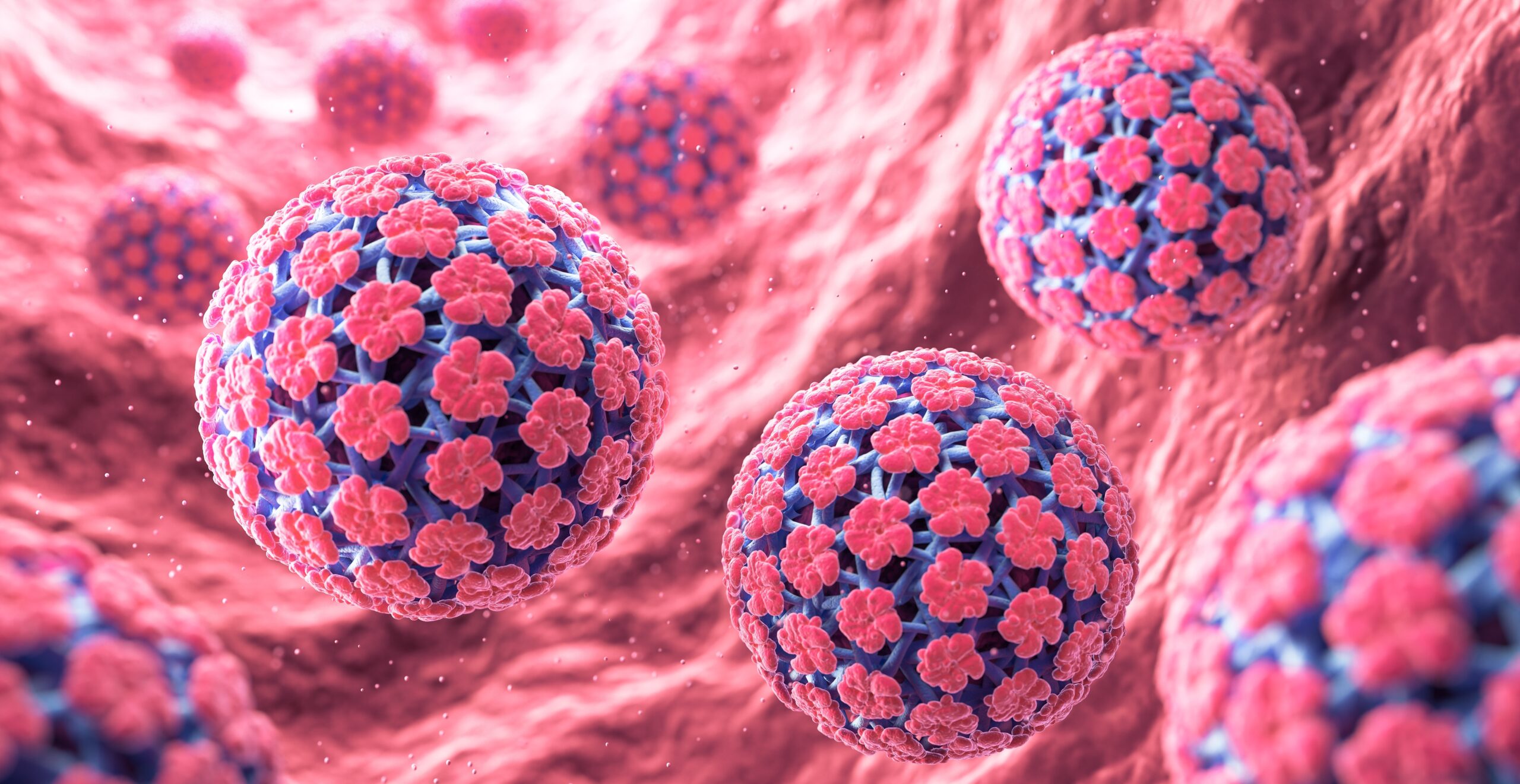
How vaccine structure shapes immune response to HPV tumours
Researchers have discovered that reorganising a single cancer-targeting peptide within a spherical nucleic acid vaccine dramatically boosts the immune system’s ability to attack HPV-driven tumours.

Over the past decade,…
Continue Reading
-

Immunexpress Announces Publication Showing SeptiCyte RAPID® Identifies Septic Patients Most Likely to Have Positive Blood Cultures
Publication highlights strong association between a patient’s sepsis risk and likelihood of positive cultures, with SeptiCyte RAPID results just 90 minutes after a blood draw
Continue Reading
-

Finding cancer cells in a cocktail of complex tissues – Sciworthy
Cancer disrupts several layers of biological blueprints, including the order of DNA sequences and chemical tags on DNA called methylation. In cancer patients, tumor samples taken directly from places like the colon or skin contain a…
Continue Reading
-

Prehospital emergency anesthesia with intubation saves lives of trauma patients
Trauma patients urgently requiring a breathing tube are more likely to survive if the tube is inserted before arriving at hospital compared to insertion afterwards, suggests a modeling study led by researchers at University College…
Continue Reading