Toward a Muscarinic Agent Solution for the Presynaptic Dopamine Problem in Schizophrenia
Research on Cobenfy (xanomeline-trospium) presents a novel approach to management of schizophrenia by targeting muscarinic acetylcholine receptors. By…

Research on Cobenfy (xanomeline-trospium) presents a novel approach to management of schizophrenia by targeting muscarinic acetylcholine receptors. By…

We are continuing our series, Media Day, where we spotlight individual medical institutions and their infectious disease (ID) programs. This episode…
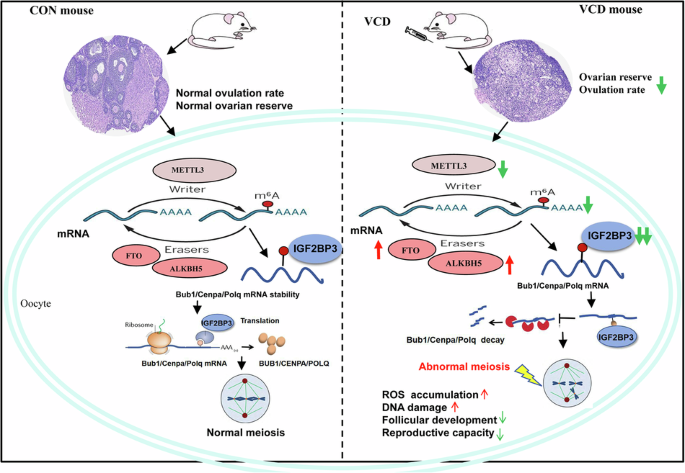
Li, Y., Li, M., Liu, J., Nie, G. & Yang, H. Altered m6A modification is involved YAP-mediated apoptosis response in 4-vinylcyclohexene diepoxide induced ovotoxicity. Ecotoxicol. Environ. Saf. 262, 115192 (2023).
Park-Lee, R. E. A. Key Substance Use and Mental Health Indicators in the United States: Results from the 2020 National Survey on Drug Use and Health. HHS Publ No PEP19-5068, NSDUH Ser H-54. 170 51–8 (2019).
El Osta, R. et al. Anabolic steroids…

You can live for years with obstructive sleep apnea and never know it. The condition repeatedly narrows your upper airway during sleep. That can break up your rest, strain your body, and lower oxygen levels overnight. Researchers have long…

In today’s screen-driven world, millions spend most of their days indoors — working, relaxing and socialising within four walls.
While this lifestyle feels convenient, growing research shows it may come at a…
This year, the International Diabetes Federation (IDF) officially recognized a fifth form of diabetes, after decades of controversy. It’s now urging other health authorities, like the World Health Organization (WHO), to follow suit.
Type 5…
Each week, Live Science highlights an intriguing case report from the medical literature, where we explore unusual symptoms, rarely seen diagnoses and out-of-the-box treatments. Through this “Diagnostic Dilemma” series, we describe how doctors…