A leading surgeon behind a clinical trial of transplanting pig kidneys into living humans has said they could one day be superior to those from human donors.
Dr Robert Montgomery, the director of NYU Langone’s Transplant Institute, said the…
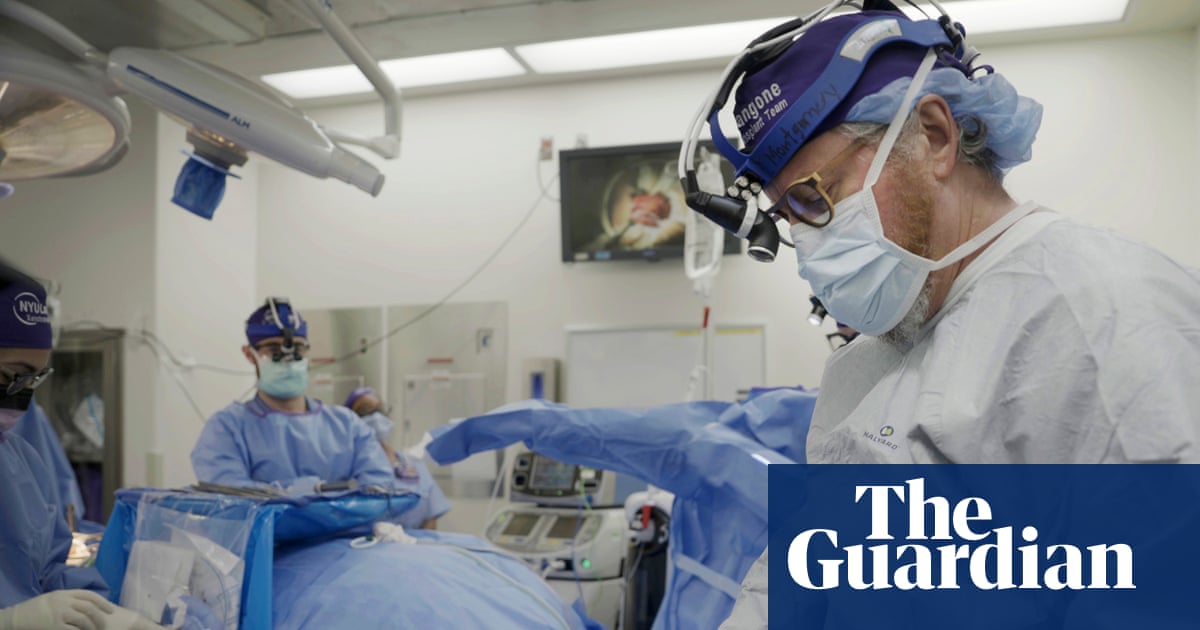
A leading surgeon behind a clinical trial of transplanting pig kidneys into living humans has said they could one day be superior to those from human donors.
Dr Robert Montgomery, the director of NYU Langone’s Transplant Institute, said the…
National Centre for disease control. Ministry of Health and Family Welfare Directorate General of Health Services, Government of India. Seasonal influenza. State/UT-wise, Year-wise number of cases and deaths from 2018–2024.
ULAN BATOR, Dec. 26 (Xinhua) — Mongolia has recorded six new cases of measles infection over the past 24 hours, bringing the national caseload to 13,752, according to the country’s National Center for Communicable Diseases (NCCD) on Friday.
…
ULAN BATOR, Dec. 26 (Xinhua) — Mongolia has recorded six new cases of measles infection over the past 24 hours, bringing the national caseload to 13,752, according to the country’s National Center for Communicable Diseases (NCCD) on Friday.
…

Scientists have identified a promising new cancer-fighting approach hidden in the gut bacteria of a Japanese tree frog, with one bacterial strain eliminating tumors in mice without causing severe side effects.
Researchers from the Japan…
Xie, T. et al. Seismic monitoring of rockfalls using distributed acoustic sensing. Eng. Geol. https://doi.org/10.1016/J.ENGGEO.2023.107285 (2023).
Chen, Z. et al. Eavesdropping on wastewater…

We recognize you are attempting to access this website from a country belonging to the European Economic Area…